For May, it’s in your bones: calcium and phosphorus
The International Year of the Periodic Table marks the 150th anniversary of Dimitri Mendeleev’s periodic system. The American Society for Biochemistry and Molecular Biology is joining the celebration with a series of articles on biochemical elements. Since January, we have presented hydrogen; iron; sodium, potassium and chlorine; and copper.
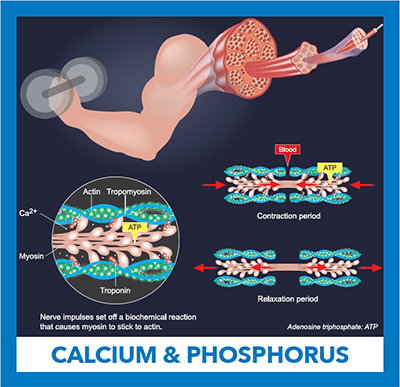 An electrical impulse traveling from a motor neuron results in the release of calcium from the muscle’s intracellular stores. Ca+2 binds to the inhibitory troponin-tropomyosin complex, allowing myosin and actin filaments to slide past one another causing muscle contraction. Adenosine triphosphate is hydrolyzed in the process. Relaxation follows when cytoplasmic calcium is removed.
An electrical impulse traveling from a motor neuron results in the release of calcium from the muscle’s intracellular stores. Ca+2 binds to the inhibitory troponin-tropomyosin complex, allowing myosin and actin filaments to slide past one another causing muscle contraction. Adenosine triphosphate is hydrolyzed in the process. Relaxation follows when cytoplasmic calcium is removed.
May is arthritis awareness month, so we selected calcium and phosphorous, the two components of the mineral salt hydroxyapatite that makes up about 65 percent of the human adult bone mass.
With chemical symbol Ca and atomic number 20, calcium is classified in the periodic table as an alkaline earth metal. In chemical reactions, calcium easily loses the two valence electrons in its outermost orbital to form ionic compounds that contain dipositive Ca+2.
At 3 percent of the Earth crust’s mass, calcium is the fifth most abundant element and the third most common metal after iron and aluminum. Most of the Earth’s calcium is found as a carbonate mineral in limestone — sedimentary rock that contains fossilized sea life. Calcium carbonate makes corals, sea shells and pearls when Ca+2 released by weathering reacts with seawater bicarbonate.
Calcium is essential in biology. Both prokaryotes and eukaryotes maintain low intracellular free Ca+2 via ion channels, transporters and calcium-sequestering proteins. In response to environmental changes, intracellular Ca+2 rapidly rises, transmitting the outside information to the interior of the cell. In bacteria, this calcium signaling system regulates chemotaxis — or movement toward a chemical stimulus — and flagellar rotation.
In mammals, cells respond to hormones by activating the phosphoinositide 3-kinase signaling pathway that leads to high intracellular Ca+2 and expression of calcium-dependent genes. Excited neurons release the neurotransmitter acetylcholine, which binds to its receptor on the receiving cell, opening ion channels and allowing the influx of extracellular Ca+2. At synapses, the inflow of calcium propagates the electrical signal to the receiving neuron, and at neuromuscular junctions, it triggers muscle contraction in the receiving fiber.
Phosphorus — with chemical symbol P and atomic number 15 — is a reactive nonmetal that combines with other elements mainly by sharing electrons via covalent bonds. Free phosphorus is rare; the element normally is found in compounds in oxidation states of +3, +5 and -3.
Phosphorus is the 11th most common element on Earth. About one gram of phosphate is found for every kilogram of the Earth’s crust, mostly in the form of oxidized inorganic rocks formed over millions of years.
Phosphorus is required for all life. Some bacteria derive energy for growth by oxidizing H2PO3– or phosphite to inorganic phosphate. Phosphate groups are major structural components of nucleotides, which are the building blocks for nucleic acids like DNA and RNA. Phospholipids — which contain a hydrophobic fatty acid “tail” and a hydrophilic phosphate “head” — form lipid bilayers that constitute cellular membranes.
Most cellular metabolic reactions are driven by chemical energy harnessed from the cleavage of adenosine triphosphate, a molecule that contains a sugar, a nitrogenous base and three phosphate groups. The addition of phosphoryl groups to proteins during phosphorylation changes protein activity and/or cellular localization, regulating a plethora of cell-signaling events.
A year of (bio)chemical elements
Read the whole series:
For January, it’s atomic No. 1
For February, it’s iron — atomic No. 26
For March, it’s a renal three-fer: sodium, potassium and chlorine
For April, it’s copper — atomic No. 29
For May, it’s in your bones: calcium and phosphorus
For June and July, it’s atomic Nos. 6 and 7
Breathe deep — for August, it’s oxygen
Manganese seldom travels alone
For October, magnesium helps the leaves stay green
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

Exploring lipid metabolism: A journey through time and innovation
Recent lipid metabolism research has unveiled critical insights into lipid–protein interactions, offering potential therapeutic targets for metabolic and neurodegenerative diseases. Check out the latest in lipid science at the ASBMB annual meeting.
Melissa Moore to speak at ASBMB 2025
Richard Silverman and Melissa Moore are the featured speakers at the ASBMB annual meeting to be held April 12-15 in Chicago.
A new kind of stem cell is revolutionizing regenerative medicine
Induced pluripotent stem cells are paving the way for personalized treatments to diabetes, vision loss and more. However, scientists still face hurdles such as strict regulations, scalability, cell longevity and immune rejection.

Engineering the future with synthetic biology
Learn about the ASBMB 2025 symposium on synthetic biology, featuring applications to better human and environmental health.

Scientists find bacterial ‘Achilles’ heel’ to combat antibiotic resistance
Alejandro Vila, an ASBMB Breakthroughs speaker, discussed his work on metallo-β-lactamase enzymes and their dependence on zinc.

