A new kind of stem cell is revolutionizing regenerative medicine
Regenerative medicine is rewriting the future of health care, with stem cells leading the charge as researchers report that their experimental induced pluripotent stem cell, or iPSC, therapeutics could restore vision and cure Type 1 diabetes.
Unlike embryonic stem cells, iPSCs are reprogrammed from adult cells to a pluripotent state from which they can differentiate into myriad cell types such as neurons, cardiomyocytes, insulin-producing pancreatic cells and more. According to experts, iPSCs have the potential to repair or replace damaged tissues and treat a range of diseases considered incurable, including Parkinson's, heart failure, spinal cord injuries and diabetes.

Constantinos Chronis, an assistant professor of biochemistry and molecular genetics at the University of Illinois Chicago, said iPSCs made from a patient’s own cells minimize the risk of immune rejection as well as other issues with traditional stem cell therapies.
The game-changer
Traditional stem cell research and therapies primarily relied on embryonic stem cells, or ESCs, derived from the inner cell mass of blastocysts in the early stages of embryonic development. Scientists began studying ESCs in the early 1980s. In 1998, U.S. scientists isolated the first human ESC, and controversy soon followed because human ESC research requires the destruction of human embryos.
“I think the U.S. is still very torn over embryonic stem cell use,” Anne Zimmerman, a bioethicist at Columbia University, said. "(It’s) reasonable to say adult cells will be able to provide some of the stem cell therapies."
ESC research laid the groundwork for modern stem cell science, including the development of iPSCs in 2006 by Shinya Yamanaka.
When researchers introduce specific transcription factors, known as the Yamanaka factors — OCT4, SOX2, KLF4 and c-MYC — into an adult somatic cell, such as a skin fibroblast, the result is an iPSC. This technique, using either chemical or virally encoded instructions, resets the cell’s identity and fate.
The clinical cutting-edge
In October 2024, researchers in China reported that they successfully restored glycemic control in a patient with Type 1 diabetes using islets derived from chemically induced iPSCs.
The team reprogrammed mesenchymal stromal cells from the patient's adipose tissue into iPSCs, which were then converted to insulin-producing pancreatic beta cell islets using growth factors and cytokines that promote pancreatic cell differentiation. After differentiation, the researchers transplanted these synthetic islets under the patient’s abdominal muscles. One year after the transplant, the patient needed no insulin injections to maintain glycemic control.

Frank Edenhofer, head of genomics, stem cell biology and regenerative medicine as well as the Molecular Biology Institute at the University of Innsbruck, Austria, said such an autologous approach, deriving beta cells from a patient’s own tissue, minimizes the risks of immune rejection. However, he said, this method will likely be “too costly to produce in a personalized manner” for a large number of patients in hospitals with standard equipment.
In addition, the initial study’s small sample size — one patient — limits any broad conclusions about safety and reproducibility. According to Chronis and Edenhofer, future research must address scalability and long-term consequences of transplantation.
Chronis pointed out that the scientists transplanted the iPSC-derived beta cells outside of their natural niche, the pancreas. Unfamiliar surroundings could negatively impact their physiological function, interactions with neighboring cells and potentially lead to tumor formation.
"This study leaves open questions about the nurturing environment,” Chronis said. “Cells don't just grow in the air, but they interact with other cells. They are in specific niches that support their function.”
Two weeks after the Type 1 diabetes study was published, researchers in Japan reported they used iPSCs to treat limbal stem cell deficiency. In this rare eye condition, corneal stem cells are unable to regenerate and repair the eye’s surface. The team in Japan differentiated human iPSCs to corneal epithelial cells and engineered them into cell sheets suitable for transplantation. Over two years, the patients’ vision sharpened, and their corneas became less cloudy.
This trial involved four patients who received the iPSC-derived corneal epithelial cells from a third-party cell line, so the transplanted cells did not genetically match the patients’ cells. Therefore, some patients had to be treated with immunosuppressive drugs to mitigate the risk of autoimmunity.
“This study uses an allogenic approach,” Edenhofer said. “You can make a big batch of cells which can then be distributed to clinical centers applying this therapy to numerous patients. That is relatively cost-efficient.”

A similar early clinical trial for limbal stem cell deficiency is ongoing at UCLA Health.
Despite their limitations, Edenhofer and Chronis agreed these studies represent important advances in the field.
“(These studies) are fantastic,” Chronis said, “in the sense that we're now talking about doing something that we envisioned maybe a decade ago or five years ago, in real patients and looking at the outcome of these cells in real time, while continuously monitoring for adverse effects.”
Elizabeth Godwin, a 41-year-old adult woman with Type 1 diabetes, said she is interested in any therapy that could restore her body's ability to produce insulin.
“Even with the intensive blood sugar management that I practice … I can't mimic the same results as naturally produced insulin with the synthetic version I have to take,” Godwin said. “It takes longer to start working, takes longer to stop working and is more unpredictable, which requires me to put in a lot of mental and practical effort to keep myself in a safe range.”
Godwin also said that a stem cell therapeutic would likely allow her to shift financial resources and mental energy to engaging with family and travelling. But she would also be wary of potential side effects, such as cancer, and whether the stem cells would remain effective for the rest of her life.
“It can feel like I am living minute-to-minute at the mercy of the insulin, which provides my only choice to stay alive,” Godwin said. “I would love to see treatment options advance beyond the incremental improvements to the current approach, which has been the standard of care for Type 1 diabetes since the first injection of insulin in the 1920s.”
Understanding what makes iPSCs tick
Because researchers can transform stem cells into a variety of cell types, Chronis underscored the need to distinguish between stem cell–derived cell behavioral and molecular characteristics.
“Being able to produce a functional cell is the goal,” Chronis said. “But, having a real molecular identity that very accurately and closely recapitulates the (bodily) in vivo counterpart is equally important."
The stem cell field is advancing far more rapidly than many anticipated, he added, but it lacks strong foundational knowledge.
“Different labs have proposed multiple transcription factor combinations, and even chemicals, for generating these reprogrammed (cells),” Chronis said. “(It) then becomes very difficult to have a standardized set of practices that everybody can work on and try to improve to increase the protocol fidelity.”
Chronis and his team are working to create a roadmap of mammalian stem cell development that scientists can use as a guide to create an iPSC that closely mimics the early stages of human ESC cell development.
"Our current research focuses on understanding how the Yamanaka factors dismantle somatic cell identities," Chronis said.
They recently converted skin fibroblasts into nonskin endodermal tissue, a type of tissue that develops into many of the body's internal organs, using a combination of the Yamanaka factors and other signals. Using this technology, researchers could bypass iPSC generation altogether, which could reduce production time, lower manufacturing costs and potential enhance safety, Chronis said.
"The full extent of fibroblast plasticity remains to be seen,” Chronis said. “But this presents an exciting new avenue for cell replacement therapy."
Stem cells in the brain
According to Edenhofer, stem cells of the nervous system represent an ideal cellular source for cell replacement therapies because they can generate the major cell types of the nervous system including neurons, astrocytes and oligodendrocytes. This plasticity could allow them to integrate into existing neural circuits to restore lost functions in patients with neurodegenerative diseases.
However, access to and utility of patient-derived neural stem cells is limited. These naturally occurring cells are difficult to harvest because they are found in the inner regions of the brain, and they have limited proliferative potential due to their genetic programming.
Therefore, Edenhofer wanted to use easy-to-reach, patient-derived cells to generate neural stem cells for replacement therapy. To meet this need, his team created a protocol to produce what they call induced neural stem cells, or iNSCs.
Edenhofer’s team generates iNSCs directly from patient dermal fibroblasts or blood cells. First, they infect the cells with a nonintegrating virus, containing a modified version of the Yamanaka factors — OCT4, KLF4, SOX2 and c-MYC. After a short incubation, the team transfers the dedifferentiating cells to a neuroinduction media containing growth and survival factors that promote proliferation and neural differentiation.
Though iNSCs are artificially constructed, Edenhofer said, they do not differ transcriptionally from their counterparts derived either from pluripotent stem cells or primary tissue.
"Pragmatically, it's much more cost efficient and faster to generate induced neural stem cells, because if you want to go via iPSCs to neural stem cells, we have to first generate the iPSCs,” Edenhofer said. “That means lots of cell culture time, lots of resources, costs, (labor) and the risk of undesired cellular changes during prolonged culture time. After that, you still have to redifferentiate those iPSCs into neural cells. We make a shortcut there, which allows us to do it four times faster."
Neuroregeneration
Edenhofer’s lab showed that iNSCs could reduce neuroinflammation and restore motor control in mice that have symptoms of multiple sclerosis, or MS, and spinal cord injury. The cells did this by reducing levels of the proinflammatory metabolite succinate in the cerebrospinal fluid after the researchers transplanted them into the mouse via intracerebroventricular injection, which bypasses the blood–brain barrier.
“In the long run, we must change the microenvironment from anti-regeneration to pro-regeneration,” Edenhofer said. “When you transplant our induced neural stem cells at the site of injury, they have an anti-inflammatory effect, meaning they recognize the proinflammatory molecules, like extracellular succinate, and suck it up like a sponge. They also activate anti-inflammatory genes to induce remyelination and neuronal rewiring.”
To use this method in humans without risking immune rejection, Edenhofer said, the iNSCs must be derived from a patient’s own fibroblasts, and a person’s illness is likely to affect those cells.
Therefore, his team recently performed a study in mice to compare dermal fibroblast–derived iNSCs from human healthy donors to those from patients with progressive MS. They found that iNSCs from MS patient fibroblasts had increased glucose-dependent fatty acid and cholesterol synthesis, which led to lipid droplet accumulation. This accumulation caused the iNSCs to upregulate inflammatory signaling and take on a senescent-like phenotype, which promoted neurotoxicity in mature neurons.
To combat this, the researchers administered the HMGCR inhibitor simvastatin to the MS patient–derived iNSCs, which reversed the hyperinflammatory phenotype; thus, reducing potential neurotoxicity.
Overcoming challenges to translation
Among the most significant challenges when working with iPSCs, are the task of efficient generation of the cells and mitigating the risk of tumors forming after they are transplanted.
Reprogramming adult cells into iPSCs is inefficient. Only a small fraction of treated cells reach the pluripotent state; studies show that fewer than 1% of treated cells successfully become iPSCs, depending on the methods used. The type of donor cell, the specific combination of reprogramming factors and the delivery system can all influence outcomes.
“Cells are problematic, in particular stem cells,” Edenhofer said. “Our novel technologies nowadays, single-cell omics, allow us to see that in apparently homologous populations, we still see a lot of heterogeneity that might be due to failed differentiation, survival or integration after transplantation."
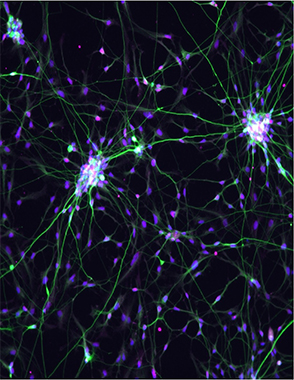
Undifferentiated iPSCs or incompletely differentiated cells can cause tumors because they retain the ability to proliferate indefinitely, Edenhofer said. To address this, scientists are exploring emerging technologies, such as CRISPR-based editing and molecular “suicide switches” that trigger the destruction of aberrant cells to prevent tumor formation.
Yet, another challenge is the large volume of cells needed for stem cell therapeutics. Unlike hematopoietic bone marrow transplants used to treat leukemia, nonhematopoietic stem cell therapeutics require hundreds of millions of cells.
“Scaling up will be an issue,” Edenhofer said. “We know from the very early days of stem cell transplantations, relatively few hematopoietic stem cells are sufficient to repopulate the complete blood system in a recipient mouse. But that is not true at all for all stem cells. For example, in the brain, as one can imagine, you cannot repopulate the nervous system with just a few neural stem cells."
As of March 2025, the U.S. Federal Drug Administration had only approved one nonhematopoietic stem cell therapeutic: Ryoncil, an allogeneic bone marrow–derived mesenchymal stem cell therapy for steroid-refractory acute graft versus host disease in pediatric patients two months of age and older.
First discovered in the 1970s, mesenchymal stem cells are multipotent cells known for their ability to modulate immune responses and promote tissue repair. These anti-inflammatory properties make mesenchymal stem cells valuable for treating diseases where the immune system is overactive, such as graft versus host disease. However, Ryoncil uses mesenchymal stem cells directly from a donor and does not involve iPSC technology.
However, a recent study identified 115 clinical trials with regulatory approval, testing 83 human pluripotent stem cell products. Most of these trials target eye diseases, central nervous system disorders and cancer.
Confronting ethical conundrums
The National Institutes of Health requires graduate students in NIH-funded research labs in the U.S. to complete a bioethics course. However, this course rarely touches on stem cell research, focusing instead on interpersonal relationships, research misconduct, data management and research using human subjects. This lack of education often leaves scientists wary of stem cell research, Edenhofer said.
Chronis stressed the importance of continued collaboration and discussion among scientists, ethicists and regulators to drive the stem cell field to meet patient medical needs.
“This research is not just being done for the sake of it, but rather to benefit humanity and to try and cure incurable diseases and bring new therapies to patients,” Chronis said. “The field is certainly much more mature than it was five years ago.”
Chronis and other researchers say that ethical concerns and outdated regulations could hamper research to advance stem cell therapeutics in the U.S. while other countries forge ahead.
"There have to be clear guidelines and clear regulations, and there are always concerns of how things are going to be used,” Chronis said. “It's better to have regulations in the open than to put pressure to stop research in one field and drive researchers to take their work somewhere else, outside the U.S."
Furthermore, critics of cellular therapies assert that they are so expensive most people could never afford them.
In March 2025, Mesoblast announced that a complete course of Ryoncil will cost $1.55 million. Edenhofer does not think the potential cost is a reason to halt research and development.
Edenhofer does not think the potential cost is a reason to halt research and development.
“If you go back to the first days of hematopoietic stem cell transplantation, they were not affordable at all, and very few people thought they would work out,” Edenhofer said. “Today, not everybody worldwide can expect to get such a treatment, but many transplantations have been carried out. So, it's definitely a success story. Why not think that new stem cell treatments can also develop this way?"
The number of stem cell transplants in the U.S. has grown exponentially over time. In the 1980s, only about 1,500 patients received this therapy compared to almost 23,000 in 2022.
Looking ahead
In 2018, the NIH spent $31 million on research using fetal tissue. In 2019, President Donald Trump signed regulations prohibiting NIH scientists from conducting such research. During the first Trump administration, policy shifts favored research on adult stem cells and induced pluripotent stem cells as less controversial alternatives.
Robert F. Kennedy Jr., the director of the U.S. Department of Health and Human Services, was asked about stem cell research during his confirmation hearing by Sen. Maria Cantwell, D-Wash, and said he would “protect stem cell research.”

The field has made significant progress using organoids, 3D self-organizing cell cultures that mimic organ structure and function, in the past decade. According to Edenhofer, and the ability to grow full-sized organs may not be far off.
“We are now at the stage where the scientists can produce or generate … early stages of human embryos in situ without implantation into the uterus,” Edenhofer said “But what does this mean for us in the future?"
Indeed, a laboratory at Columbia University is creating lifelike, 3D skin gloves using foreskin-derived neonatal fibroblasts and keratinocytes to treat burn victims.
Bioethicist Zimmerman is unsure about the ethical implications of reproducing complex organs.
"I just don't think, as a country, that we are ready for (scientists) to grow a brain in a lab,” Zimmerman said.
However, Chronis and Edenhofer underscored the need to keep pushing the field forward while balancing ethical concerns.
“The stem cell field is very young,” Chronis said. “And there is a fantastic future for it.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Finding a symphony among complex molecules
MOSAIC scholar Stanna Dorn uses total synthesis to recreate rare bacterial natural products with potential therapeutic applications.

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

