JLR: Secrets of fat and the lymph node
When you eat a high-fat meal, your gut exports the fat into chylomicrons, which join the flow of lymph in the surrounding vessels. This combined fluid, which resembles cream, passes rapidly through the lymph nodes and into the blood stream, where the fat is absorbed by cells that need energy, like heart muscle cells, or that can store fat for the long term, like adipose tissue.
Sander Kersten, a professor and department chair at Wageningen University in the Netherlands, studies that rapid uptake system, which depends on a protein called lipoprotein lipase, or LPL for short. LPL breaks down triglyceride fat molecules, allowing them to be absorbed. Kersten’s latest study in the Journal of Lipid Research shows that LPL regulation varies among tissues.
Too much fat in the blood can cause problems such as heart disease. Therefore, right after a meal, it is beneficial for adipose tissue to absorb fat rapidly for storage. That means bumping up LPL levels. But during a long fast, limited LPL activity in adipose tissue helps ensure that fat stays available to cells that need it for energy. It is important that our bodies can tune LPL activity in different systems in response to how much food we eat.
Some 20 years ago, as a postdoc, Kersten isolated a protein, ANGPTL4, that acts as a control dial for LPL. Later, his lab demonstrated that the protein targets LPL for degradation in adipose cells. Researchers since have found that ANGPTL4 fluctuates in response to fasting, cold exposure and exercise, helping to control the body’s lipid use.
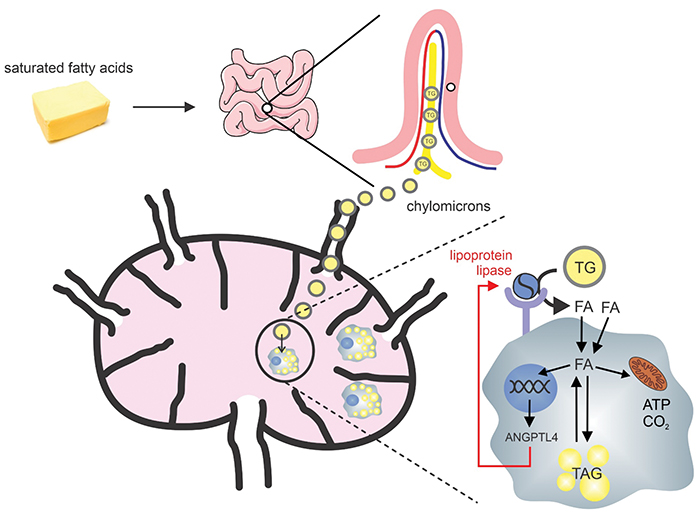 A cartoon Kersten uses for teaching shows how saturated fat from the diet enters the lymph node as chylomicrons and how lymph node resident macrophages respond to the fat.Courtesy of Sander KerstenDrug developers hoped that reducing ANGPTL4 would be a good way to reduce the risk of heart disease, but this research hit a snag. Mice that were bred to have no ANGPTL4 appeared healthy at first, but that health was fragile.
A cartoon Kersten uses for teaching shows how saturated fat from the diet enters the lymph node as chylomicrons and how lymph node resident macrophages respond to the fat.Courtesy of Sander KerstenDrug developers hoped that reducing ANGPTL4 would be a good way to reduce the risk of heart disease, but this research hit a snag. Mice that were bred to have no ANGPTL4 appeared healthy at first, but that health was fragile.
“If you place these animals on a diet that’s rich in fat, they develop complications which were unanticipated,” Kersten said. Lymph carrying chylomicrons escapes into their abdomens, eventually killing the mice. Whether this would happen in humans if you blocked their ANGPTL4 isn’t known — it’s an experiment no one is willing to risk.
The Kersten lab’s latest paper examines why loss of ANGPTL4 has this effect. The work focuses on macrophages, the cells that populate the lymph node. Like fat cells, macrophages express ANGPTL4, and like fat cells, they turn it up in response to high fat in the bloodstream. But ANGPTL4 in macrophages appears to work differently than in fat cells. Although ANGPTL4 reduces LPL activity and fat uptake in macrophages, it doesn’t seem to alter LPL level — suggesting that it does not act by targeting LPL for degradation but by another mechanism. Exactly how ANGPTL4 affects macrophages, Kersten said, remains to be determined.
“After 20 years of studying ANGPTL4, there are some things that are very, very clear about this protein,” Kersten said. “And I’m happy to have contributed to that.”
Other questions remain. “We’re not still 100% sure about what is going on in the lymph nodes.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Tiny laboratories that fit in your hand can rapidly identify pathogens using electricity
Pathogens have distinct electrical charges, shapes and sizes. Measuring how quickly they move through an electric field can help researchers separate different species in a sample.
Toxoplasma gondii parasite uses unconventional method to make proteins for evasion of drug treatment
This recent study by a team from Bill Sullivan’s lab at the Indiana University School of Medicine was named a Journal of Biological Chemistry Editor’s Pick.

Of genes, chromosomes and oratorios
Jenny Graves has spent her life mapping genes and comparing genomes. Now she’s created a musical opus about evolution of life on this planet — bringing the same drive and experimentalism she brought to the study of marsupial chromosomes.
Ubiquitination by TRIM13: An ingredient contributing to diet-induced atherosclerosis
Researchers help unravel the molecular mechanism behind plaque formation in cardiovascular disease.

When ribosomes go rogue
Unusual variations in the cellular protein factory can skew development, help cancer spread and more. But ribosome variety may also play biological roles, scientists say.

New discovery enables gene therapy for muscular dystrophies, other disorders
At the University of Rochester, researchers find that RNA-based technology facilitates effective use for difficult-to-treat, large-gene diseases.

