For April, it’s copper — atomic No. 29
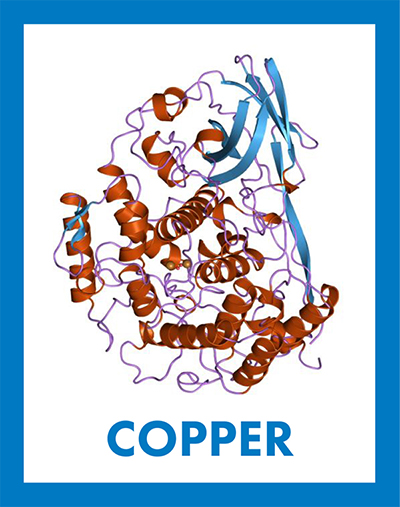
Oxygen-bound copper, represented by the two orange spheres in the center of the structure of the protein hemocyanin, gives arthropod hemolymph a blue color. Jawahar Swaminathan & MSD staff / European Bioinformatics Institute 2019 marks the 150th anniversary of the first publication of Dimitri Mendeleev’s periodic table of chemical elements. To honor the occasion, each month we are focusing on elements important in biochemistry. In January and February, we featured hydrogen and iron, respectively, and in March, we tripled down with sodium, potassium and chlorine.
For April, we have selected copper, a transition metal with chemical symbol Cu and atomic number 29. Copper participates in chemical reactions via a wide range of oxidation numbers from -2 to +4 but most commonly is found in organic cuprous and cupric complexes with oxidation states of +1 and +2, respectively.
Ancient civilizations used copper extensively in the manufacture of ornaments, weapons and tools, and the late fifth millennium BC is the archeological period known as the Copper Age.
Copper most likely forms during the expansion of supergiant stars and makes up about 0.0068 percent of the Earth’s crust. In nature, it occurs in its pure metallic form as native copper or in combination with other elements in minerals containing copper oxides, copper sulfides or copper carbonates. These minerals form when volcanic activity separates copper from magma and the copper reacts explosively with ascending sulfurous gases, precipitating beneath the surface as copper-rich ores.
After iron and zinc, copper is the third most abundant metallic element in biological systems. Copper is indispensable for most living organisms in trace amounts, but it can produce highly reactive toxic species when found in excess inside cells. Microorganisms can mobilize solid copper by incorporating it into cyanide compounds, and copper-binding proteins in the periplasmic space allow bacteria to trap copper to avoid intracellular toxicity. In yeast, cell surface metalloreductases render copper available to high-affinity transporter proteins by reducing Cu+2 to Cu+1. Fungi that colonize the roots of host plants protect them from toxicity by trapping soiled or soluble copper in compounds with other metals.
Once in cells, copper binds to proteins that participate in electron transfer reactions or oxygen transportation. In the mitochondrial respiratory chain, a binuclear copper center in cytochrome c oxidase transfers electrons from cytochrome c to molecular oxygen in the final redox step of the oxidative phosphorylation pathway. Similarly, a copper-containing protein called plastocyanin in the thylakoid lumen of chloroplasts carries electrons between two membrane-bound proteins — cytochrome f and P700+ — during photosynthesis.
Like hemoglobin in vertebrates, a copper-containing protein called hemocyanin transports oxygen in the hemolymph of invertebrates, such as mollusks and some arthropods. When a molecule of oxygen binds two copper atoms in hemocyanin, copper is oxidized from Cu+1 to Cu+2, and the energy associated with this electron transfer changes the light-absorbing properties of copper. This causes oxygen-rich hemolymph in these animals to appear blue in contrast to the bright red color of oxygen-bound iron in hemoglobin.
In eukaryotes, the antioxidant enzyme superoxide dismutase uses copper to catalyze the decomposition of the superoxide radical into molecular oxygen and hydrogen peroxide, which are less toxic to the cell.
Thus, from the heart of volcanoes to the relay of electrons inside cells, copper is critical to the survival of all aerobic organisms.
A year of (bio)chemical elements
Read the whole series:
For January, it’s atomic No. 1
For February, it’s iron — atomic No. 26
For March, it’s a renal three-fer: sodium, potassium and chlorine
For April, it’s copper — atomic No. 29
For May, it’s in your bones: calcium and phosphorus
For June and July, it’s atomic Nos. 6 and 7
Breathe deep — for August, it’s oxygen
Manganese seldom travels alone
For October, magnesium helps the leaves stay green
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.
Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

