JLR: New insights into treating amoebic keratitis
The human body provides a hospitable environment for many micro-organisms that are essential to our survival. At the same time, it also attracts a host of parasites that, if not treated properly or eradicated, can be extremely harmful to our health. One such class of parasite is the infective amoeba, which causes rare and sometimes fatal diseases in humans. The Acanthamoeba species, found worldwide, mostly in water and soil, causes amoebic keratitis, or AK — an eye infection of the cornea that can result in permanent blindness. In the USA, 85 percent of AK cases occur in soft contact lens users. Although AK is potentially life-threatening, its treatment is not yet promising, owing to drug resistance and the absence of species-specific drugs. Hence, we need to identify specific drug targets to better fight these parasites.
Designing species-specific drugs requires an understanding of the unique evolutionary differences among species, especially with respect to biochemical pathways responsible for the survival of the parasite within the host. The Acanthamoeba life cycle has two stages — cyst and trophozoite. The trophozoite is the active form that infects humans, while the cyst is the dormant form that can survive harsh conditions such as stress and lack of nutrients. When conditions become favorable, the cyst transforms to a trophozoite via a process called excystment. Both forms can enter the human body through wounds, nostrils or contact with water.
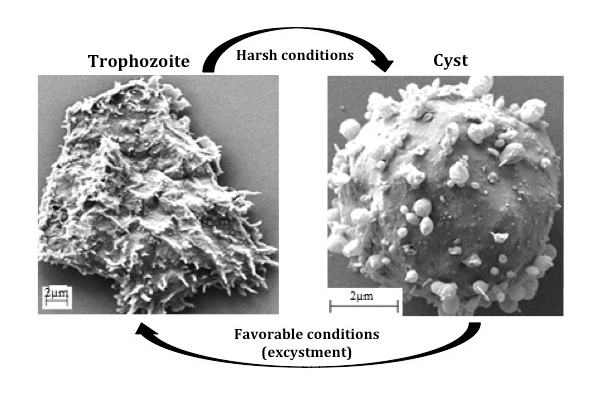 Electron micrographs show the two stages in the life cycle of Acanthamoeba castellani. Courtesy of the W. David NES labORATORY
Electron micrographs show the two stages in the life cycle of Acanthamoeba castellani. Courtesy of the W. David NES labORATORY
Certain metabolic pathways cause the Acanthamoeba to cycle between stages and help the infective trophozoites survive and proliferate in humans. Thus, targeting these specific pathways could prove to be an efficient strategy to treat Acanthamoeba infections. W. David Nes and his group at Texas Tech University have investigated such pathways and reported sterol C24-methyltransferases, or SMTs, synthesized only in amoebae, as novel druggable targets. Their findings were published in the Journal of Lipid Research.
Sterols are amphipathic molecules that, by virtue of their lipid-based properties, act as membrane inserts to control overall growth and development. Ergosterol biosynthesis has been established as essential for the survival of many amoebae in humans, and SMTs are critical enzymes in the ergosterol biosynthesis pathway. SMTs catalyze a crucial step in the ergosterol pathway that maintains trophozoite growth. Interestingly, SMTs are absent in humans. Thus, the researchers found that inhibiting these enzymes with transition-state analogs that blocked the catalytic site on the enzyme, or with suicide substrates that irreversibly bound covalently to the enzyme, stopped the growth of trophozoites but had no effect on normal cholesterol biosynthesis in human cells. So this approach could treat specifically Acanthamoeba infections without harming us. This is the highlight of Nes’ published work.
The work has been quite challenging, especially because differences in sterol biochemistry and life-cycle events among amoeba species make it hard to identify common drug targets. Moreover, Nes’ group required an extensive collaboration to integrate a multidisciplinary approach so as to provide “the most effective drugs, which would escape mechanisms that otherwise could compromise their therapeutic longevity,” Nes said.
Having used keratitis-causing Acanthamoeba castellanii as the model system in their published study, Nes and his group now want to test their hypothesis in mouse models. They also plan to extend their inhibitor studies to Naegleria fowlerii, a “brain-eating amoeba” that can cross the blood-brain barrier and destroy brain tissue, resulting in a disease called primary amoebic meningoencephalitis, or PAM. Further down the road, they hope to develop high-throughput screening techniques to repurpose existing drugs as novel SMT catalysis inhibitors to cure amoebic infections.
 Isha Dey is a Ph.D. candidate at Rosalind Franklin University of Medicine and Science.
Isha Dey is a Ph.D. candidate at Rosalind Franklin University of Medicine and Science.Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Of yeasts and men: One-hour proteomes, 10 years apart
To profile the human genome within an hour, the researchers used a new mass spectrometer and packed their liquid chromatography columns with very high pressure.
Cells have more mini ‘organs’ than researchers thought
Membraneless organelles, also called biomolecular condensates, are changing how scientists think about protein chemistry, various diseases and even the origin of life.

Institute launches a new AI initiative to power biological research
Stowers investigator Julia Zeitlinger selected to head effort and leverage cutting-edge computational techniques to accelerate scientific discoveries.
From the journals: JLR
Fixation method to quantify brain metabolites. Belly fat and liver disease crosstalk. Stopping heart diseases in schizophrenic patients. Read about the recent JLR papers on these topics.

Does a protein hold the key to Alzheimer’s?
Researchers in Maryland and Massachusetts team up to study how SORL1 promotes tau trafficking and seeding in cells that leads to the neurodegenerative disorder.

Cracking the recipe for perfect plant-based eggs
It involves finding just the right proteins. With new ingredients and processes, the next generation of substitutes will be not just more egg-like, but potentially more nutritious.

