Uncovering details of molecular Ferris wheels inside cell structures
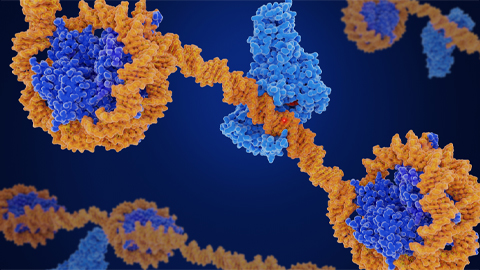
Using the Summit supercomputer at the US Department of Energy's Oak Ridge National Laboratory, a team at the Arizona State University Biodesign Institute has confirmed details of a molecular mechanism that cell organelles use to regulate the pH of their environment — and it looks a lot like a Ferris wheel.
Led by Abhishek Singharoy, a researcher at the Biodesign Center for Applied Structural Discovery (CASD) and Arizona State University's School of Molecular Sciences (SMS), the team used Summit to simulate molecular pumps that are embedded in the fatty outer membranes of cellular organelles, or cell structures that are specialized to perform different tasks for the cell's functioning. With rotors that spin 100 times per second, these pumps rapidly move protons — positively charged subatomic particles — into a cell's organelles to maintain the acidic environment inside that is crucial to the work they perform.
Using the nation's fastest supercomputer — the Oak Ridge Leadership Computing Facility's (OLCF's) Summit — the team uncovered a key step in how this mechanism works in one proton pump in particular, a yeast proton pump called vacuolar ATPase (V-ATPase) that is necessary for a variety of tasks in the body and has implications in disease. The results were published in Science Advances.

"The V-ATPase proton pumps perform a wide range of functions, from helping transmit nerve signals to helping specialized cells secrete acid for maintaining bone," said Stephan Wilkens, a biochemist at SUNY Upstate Medical University and study co-author. "Malfunctions in these molecular machines contribute to diseases such as osteoporosis, neurodegeneration, diabetes, cancer, and AIDS, so understanding them is important for human health."
A team led by Wah Chiu—professor at the SLAC National Accelerator Facility and Stanford University and codirector of the Stanford-SLAC Cryo-EM Facilities—together with Wilkens and SLAC/Stanford postdoctoral researcher Soung-Hun Roh, had already performed work at SLAC that employed Cryogenic electron microscopy (cryo-EM) to study these proton pumps. Images from the work showed 10 amino acid "seats" on a molecular Ferris wheel that bind protons and carry them through the membrane to the organelle's interior, as well as other amino acids that catch them when they arrive. Based on that picture, the team working on the research suggested that the proton drop-off might be aided by water molecules—but their images were not sharp enough to confirm that the water molecules were there. In the same study, another round of even higher resolution cryo-EM images showed the water molecules around the suspected proton path.
Singharoy and his team used the Nanoscale Molecular Dynamics, or NAMD, code to perform simulations on the Summit supercomputer and confirmed that the experimentally observed water molecules line up to form "wires" at the proton drop-off point. These wires convey protons from their seats on the Ferris wheel to landing spots inside the organelle, like a fire brigade passing buckets hand to hand, bridging a gap they couldn't navigate on their own. The work was performed under the Innovative and Novel Computational Impact on Theory and Experiment, or INCITE, program at the OLCF.
The simulations matched the cryo-EM images nicely, providing strong evidence that the picture they painted of the proton drop-off is correct.
"Molecular motors exemplify some of the most intricate chemo-mechanical devices, and our team in SMS and CASD has developed highly sophisticated computational tools to address the energy source and sinks of the motor's ratcheting motion," Singharoy said. "In 2017 we started working on the soluble part of the V-type motor, namely, V1 ATPase. Now that we have a good control on the transmembrane Vo motor, it's a great step forward toward simulating the entire motor in collaboration with Hun, Stephan, and Wah."
Chiu said that recent advances in cryo-EM that allow imaging of individual particles at atomic resolution—even when they take slightly different shapes—will open new opportunities for using it as a tool to discover effective drugs for illnesses involving proton pumps.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
CRISPR epigenome editor offers potential gene therapies
Scientists from the University of California, Berkeley, created a system to modify the methylation patterns in neurons. They presented their findings at ASBMB 2025.

Finding a symphony among complex molecules
MOSAIC scholar Stanna Dorn uses total synthesis to recreate rare bacterial natural products with potential therapeutic applications.

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

