Examining mechanisms of protein complex at a basic cell biological level
A new study highlighting the importance of a large protein complex called the exocyst in cell growth, division and communication reveals new functions and mechanisms that are essential to how molecules move across a membrane through vesicles in a cell.

Understanding how these mechanisms work in normal cells at the basic biological level will inform future research into how those functions are disrupted in developmental and neurological disorders.
“It’s the first time a role for membrane fusion has been described for this complex and it’s a breakthrough in how we think about the way the exocyst complex works,” said Mary Munson, professor and vice chair for diversity in the Department of Biochemistry & Molecular Biotechnology, associate vice provost for equity in science in the Office of Health Equity and a co-corresponding author on the study published in Nature Structural & Molecular Biology. The research was done in collaboration with Tae-Young Yoon, professor of biological sciences at the School of Biological Sciences and Institute for Molecular Biology and Genetics at Seoul National University in Seoul, South Korea.
“Until our collaborative study, exocyst was understood to recognize and possibly tether secretory vesicles to the cell membrane, prior to exocytosis. In this study, we reveal biophysical studies that indicate exocyst playing several critical roles that directly facilitate fusion of the vesicles with the cell membrane to drive cargo delivery,” Munson said.
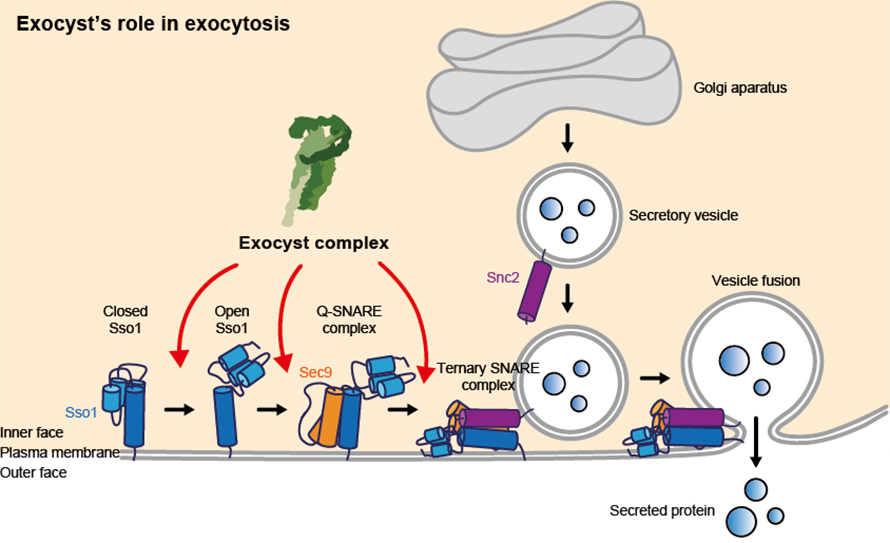
Robust control of exocytosis is critical for all cells to grow, divide and communicate properly. Dysfunction of exocyst regulation has been linked to many physiological problems in different organisms.
“It’s really an exciting development in our field, as the exocyst complex had not previously been shown to directly control vesicle fusion,” Munson said. “The complex recognizes the right vessel, the right place on the cell surface and the right time for the cargo to be delivered. It does that by talking to the membrane fusion protein.”
In 2023, Munson received a National Institutes of Health award for $3.3 million over five years to support this research.
Munson was recently named a 2024 recipient of a Zenith Award from the Association for Women in Science. The award honors senior career professionals with a lifetime of innovative achievements in STEM and a commitment to workplace diversity.
This article is republished from the UMass Chan Medical School News. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
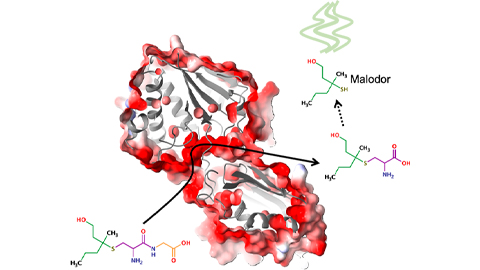
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.
Proteomic variation in heart tissues
By tracking protein changes in stem cell–derived heart cells, researchers from Cedars-Sinai uncovered surprising diversity — including a potential new cell type — that could reshape how we study and treat heart disease.

Parsing plant pigment pathways
Erich Grotewold of Michigan State University, an ASBMB Breakthroughs speaker, discusses his work on the genetic regulation of flavonoid biosynthesis.

