CRISPR epigenome editor offers potential gene therapies
Epigenetics explores how behaviors and environmental factors can change gene expression without altering the DNA sequence. In a new study, presented at the American Society for Biochemistry and Molecular Biology 2025 Annual Meeting, researchers designed CRISPR-based tools to edit neuron epigenomes, focusing on DNA methylation. This breakthrough could advance personalized treatments for neurological disorders by accounting for both genetic and environmental factors.

Neuronal genomes can be modified by the addition of a methyl group, or mCH, which is critical for gene regulation. DNA methylation occurs not only in cytosine-guanine, or CpG sites, but also at other cytosine sites, known as CpH or non-CpG methylation. CpH methylation refers to methylation at cytosines that are followed by adenine, thymine or another cytosine. Both CpG and CpH methylation sites are important for brain development and function. When these methylation patterns are disrupted, they are linked to neurodevelopmental disorders like Rett Syndrome. However, scientists do not understand how epigenetic changes within these sites lead to disease.
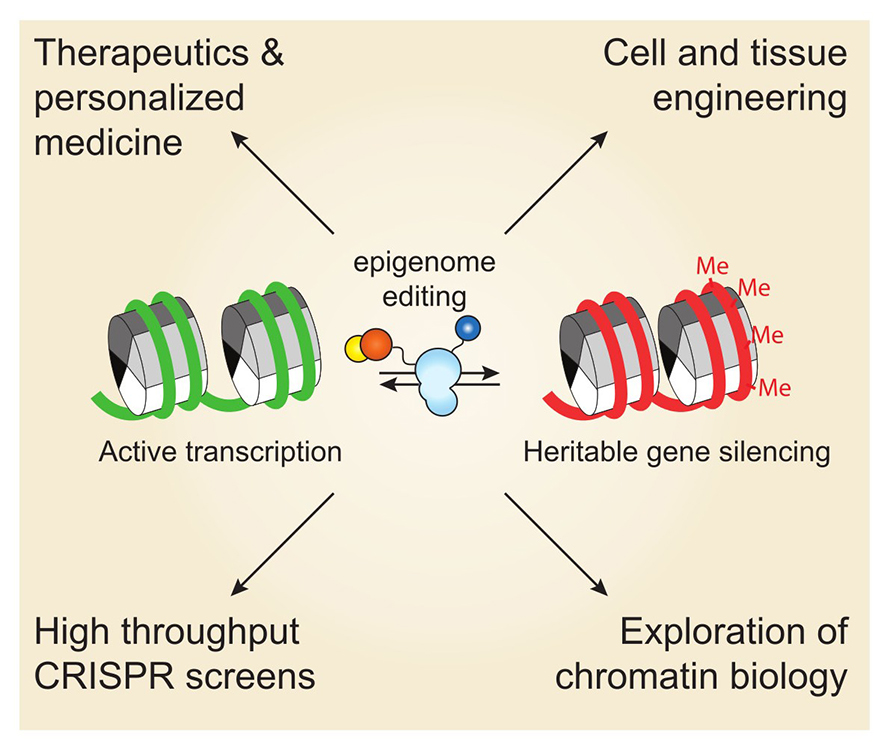
Bernardo Moreno, an undergraduate in James Nuñez’s lab at the University of California, Berkeley, is using the genome editing system CRISPR and dCas9, a catalytically dead version of the Cas9 enzyme, to alter genomic loci in neurons without cutting DNA. The system uses guide RNAs to direct dCas9, DNA methyltransferase 3A, or DNM3TA, or ten-eleven translocation, or TET, to make precise epigenetic modifications at the site of interest. DNMT3A silences genes by adding a methyl group, while TET removes these marks to reactivate expression.
Depending on whether the researchers want to silence or reactivate a gene dictates which protein, DNM3TA or TET, they fuse to dCas9. The resulting preformed protein enables targeted and reversible control of gene activity by adding or removing methylation at specific sites.
Unlike DNA or RNA-based delivery systems, which may cause lasting off-target effects, protein-based delivery systems have a short half-life, making them safer. Delivering CRISPR epigenome editor proteins with virus-like particles, or VLPs, minimizes the risk of any unintended side effects.
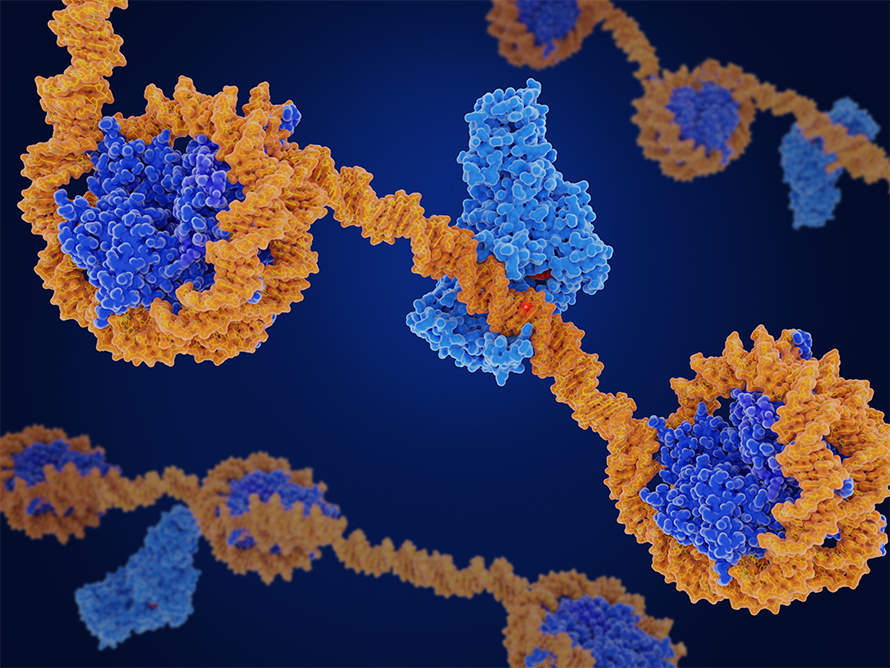
“(W)e didn’t expect this to work in neurons, especially cultured neurons, because these are traditionally very hard for reagents to get into with non-viral delivery methods,” Nuñez, an assistant professor at UC Berkeley, said.
Neurons are less responsive to common delivery methods like electroporation and chemical transfection. By using VLPs to encapsulate the large protein payload, the team achieved efficient delivery, and their platform is the first to deliver CRISPR epigenome editors as proteins.
Moreno will present his work on rewiring DNA methylation in neurons derived from induced pluripotent stem cells, or iPSCs, at ASBMB 2025 on April 14 in Chicago.
One of the team’s targets includes tau, a protein that can form neurofibrillary tangles and cause Alzheimer’s disease. Their goal was to use the CRISPR–dCas9– DNMT3A to silence the gene that encodes tau. After delivering the epigenome editor to iPSCs-derived neurons, they observed significantly lower levels of tau at both days and 14 days after treatment.
In the future, the team plans to introduce mutations in the epigenetic editor to simulate neurodevelopmental disorders and their methylation dynamics. Their long-term goal is to apply the method in animal models and eventually in humans.
“The same strategy could be applied to knock down PCSK9 gene (which drives low-density lipoprotein production) in hepatocytes to reduce cholesterol,” Moreno added, pointing to broad therapeutic potential and an interest within the biotechnology industry in translating to epigenetic-based therapies.
“There are several companies that have started epigenetic editing, which is very exciting for our field,” Nuñez said. “And now there are mice and non-human primate models showing that these tools can go into a mammal and target disease-relevant genes without ever altering the DNA sequence. It is completely epigenetics — so it’s a very exciting time.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Finding a symphony among complex molecules
MOSAIC scholar Stanna Dorn uses total synthesis to recreate rare bacterial natural products with potential therapeutic applications.

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

