
The future of fighting flu
In what an epidemiologist would consider a perfect flu season, the strains that the current influenza vaccine protects against would match perfectly those strains in circulation. If a person got a flu shot and had the misfortune to walk into someone else’s virus-laden sneeze cloud, they’d only be sick a few days rather than a week and would experience milder muscle aches and fever than without it. But when a pandemic strain emerges or one of the circulating strains mutates, the vaccine does little to protect the infected.
This happened last flu season, when the targeted H3N2 strain mutated beyond the protective abilities of the vaccine. Recent estimates confirmed that the 2017-2018 season was exceptionally dire — according to the Centers for Disease Control and Prevention, around 80,000 people died in the U.S., compared with 12,000 to 56,000 per year in other recent years.

Anthony Fauci, director of the National Institute of Allergies and Infectious Diseases, said the H3N2’s mutation was only part of the problem.
“We had a vaccine that, against H3N2, was only 25 percent effective, and we had a virus that was particularly virulent,” Fauci said. “You put those two things together, and you wind up getting a season in which you have a lot of hospitalizations and more deaths than we’ve seen in a very long time in a seasonal flu context.”
Additionally, every few decades a new strain of influenza emerges against which the human population has no pre-existing resistance, causing a flu pandemic. In 1918, crowded wartime environments set the stage for a worldwide, multiwave flu pandemic that infected an estimated 500 million people and left 50 million dead, with 675,000 deaths in the United States. At the time, the influenza virus had yet to be isolated, let alone incorporated into a vaccine, and antivirals were several decades away. With medical science in its infancy, public health measures were the strongest tools governments could bring to bear.
The most recent pandemic occurred in 2009, when a strain of H1N1 that became called “ swine flu” spilled over from pig populations, hosts for several flu strains, to humans in North America. It infected 60.8 million people in the U.S., hospitalized more than 270,000 and killed more than 12,000. While millions of doses of vaccine for H1N1 were manufactured by industry, purchased by the federal government and distributed free of cost, the vaccine doses took months to produce, ultimately arriving late in the pandemic.
If a flu vaccine worked against every potential strain of the virus, however, the 2009 pandemic and last season might have played out differently. The idea of such a universal vaccine has been gaining traction among researchers who study infectious disease; in February, the NIAID published a blueprint for the development of a universal flu vaccine in the Journal of Infectious Diseases.
As flu season gears up again, a handful of labs, including several at the NIAID and Mount Sinai Hospital, have candidates for a universal flu vaccine in various stages of development, and a number of private industry and public university researchers are attempting to improve on existing vaccine formulations.
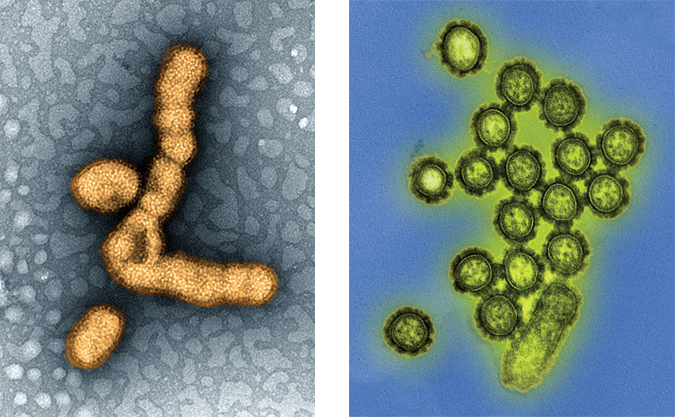
Drift and shift
Influenza’s structure and status as an RNA-negative virus make it friendly to the mutations that let it evade vaccines. If you slid samples of the virus into an electron microscope, you’d likely see spheres bristling with lollipoplike extensions, the tops of which are made out of either hemagglutinin or neuraminidase. These spherical proteins are the keys the virus uses to get in and out of cells in its host organism respectively and to which the Hs and Ns in flu virus names correspond, as in H1N1.

The viruses come in four major categories — A, B, C and D — but influenza B viruses do not circulate in animals, C viruses are believed to cause only mild respiratory illnesses in humans and D viruses affect only cattle. The flu vaccines produced every year and approved for distribution by the Food and Drug Administration are designed to protect against two A strains (an H1N1 and an H3N2) and one B strain, with some quadrivalent formulations protecting against an additional B strain.
The strains change every year because influenza is highly prone to mutations caused by errors during viral replications. While these mutations happen frequently within a flu season, the changes are usually minor enough that the existing flu vaccine protects against the new viruses in the short term. Over the course of a flu season like last year’s, however, this process, known as antigenic drift, can change a virus beyond the protective capabilities of the vaccine.

On rare occasions, influenza A viruses mutate in a more nefarious manner. Antigenic shift occurs when two viral strains with starkly different hemagglutinin sequences coinfect a host and swap genetic material, which is what happened in pigs in 2009. According to Barney Graham, deputy director of the NIAID’s Vaccine Research Center, this process is made possible by the influenza virus’ segmented genome and amplified by the flight patterns of migratory birds, a major reservoir of many flu subtypes.
Influenza “has eight gene segments that encode influenza virus protein, and because there are so many types of influenza virus and so many influenza viruses carried by migratory birds, these influenza viruses can drop into places where more than one virus can infect the same cell,” Graham said.
“There is such a large zoonotic pool in pigs and birds that influenza viruses will never be eradicated, so we have to cope with that diversity.”
Stalking heads
One of the universal vaccine candidates designed to tackle that viral diversity was developed at the Vaccine Research Center in Graham’s lab on the National Institutes of Health campus in Bethesda, Maryland. The vaccine uses self-assembling nanoparticles made of the iron-containing protein ferritin to display a conserved portion of the stalks that connect to all influenza head proteins. This elicits antibodies that can interact with a plethora of influenza strains.
As effective as these antibodies may prove to be, however, they still run up against immunodominance, a problem caused by the body’s repeated exposures to influenza antigens.
After the body is first exposed to influenza, it tends to mount future immune responses that are biased against antigens from the initial infection, NIAID director Fauci said. “It’s called imprinting, or original antigenic sin, and it tends to complicate the nature of the immune response that you have against an influenza vaccine or an influenza virus.”
This immune response means that subsequent infections and vaccinations against flu antigens cause the body to build up immunity against the parts of the flu virus that are no longer relevant to the strains it may encounter, such as older hemagglutinin proteins.
So to improve their vaccines, the researchers decapitated them.
“We can design headless, trimeric stem antigens, put them on nanoparticles and get very good antibodies against the stem region,” Graham said.
In a 2015 paper in the journal Nature Medicine, Graham and colleagues reported that this type of nanoparticle vaccine made with the stem of an H1 influenza virus could partially protect ferrets, the preferred model organism for human respiration, and completely protect mice against a lethal strain of H5N1.
The researchers also tackle immunodominance by attaching a variety of hemagglutinin molecules onto the same nanoparticle, which increases the chance for a cross-reactive antibody response; or by accumulating breadth against the various hemagglutinin subtypes by administering individual hemagglutinin-bearing nanoparticles serially.
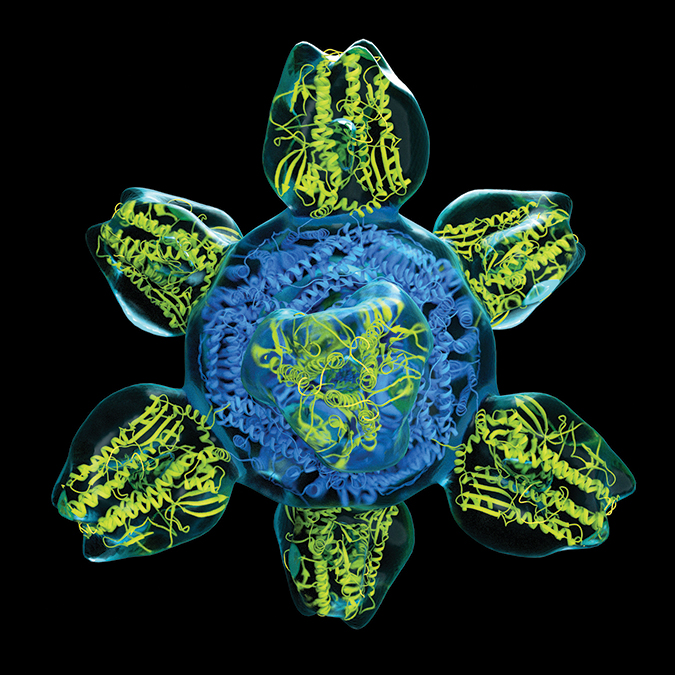
hybrid consisting of a protein scaffold (blue) and eight influenza hemagglutinin proteins (yellow).
“These new ways of displaying and designing proteins have really opened up a lot of new options for making these vaccines,” Graham said. He and colleagues have a nanoparticle vaccine using a full-length hemagglutinin that has just completed enrollment in a clinical trial and intend to bring a headless stem hemagglutinin vaccine to early clinical trials next spring, he said.
Trials of the chimera
Another promising universal flu vaccine candidate that targets the conserved stalk regions is being developed at the Icahn School of Medicine at Mount Sinai in New York by the labs led by Peter Palese, Florian Krammer and Adolpho García-Sastre.

“We already have very good vaccines against flu,” said Palese, a veteran of influenza research and vaccine development. “They are underappreciated, underutilized, but they are certainly much better than their reputation.”
To quiet the body’s immune response against the hemagglutinin heads in their vaccine, the Mount Sinai researchers developed chimeric flu proteins that consist of the conserved stalk region and artificial protein heads only found in birds, which the immune system ignores.
“What we are doing is redirecting the immune system toward these conserved regions,” Palese said.
Their universal flu vaccine is being evaluated in two clinical trials using a live attenuated inhalable platform and a traditional injectable platform, respectively backed by the Bill & Melinda Gates Foundation and GlaxoSmithKline.
In the universal flu vaccine trials, recipients are inoculated with an initial chimeric vaccine followed by a secondary chimeric vaccine with a slightly different head group; both heads will be overlooked by the body’s immune response.
In the trial of 100 volunteers funded by the Gates Foundation, the nasal spray vaccine is being evaluated for safety. In the 400-patient trial backed by GSK, the injectable vaccine is being evaluated for safety and its ability to induce an immune response. In more extensive future trials, the efficacy and potential adverse reactions of the vaccines would be monitored in as many as 3,000 volunteers for one to four years.
“I can tell you that we have not heard, either for the Gates Foundation trial or for the GSK trial, whether there are any safety issues,” Palese said. “We would have heard that, so I think that looks very good.”
Both trials are also taking samples to evaluate the optimal schedule for immunization, including the number of doses a patient might need over several months. This information ultimately may help researchers determine the duration of the vaccine’s cross-protection, or how long a patient would be protected against circulating flu strains.
“I’d like to see a lifetime kind of approach,” Palese said. “For example, if one gets infected with an influenza virus in 2018, there is very good evidence that that protection lasts for the entire life against that particular strain.”
Against the strain
In August, a paper published in Nature Communications by researchers at the University of Pennsylvania’s Perelman School of Medicine led by Scott Hensley and Drew Weissman found that it was possible to elicit an immune response against a number of hemagglutinin stems in mice and ferrets by using a vaccine made of messenger RNA encoded in lipid nanoparticles.
Another vaccine candidate, developed by Jeffrey K. Taubenberger at the NIAID’s Viral Pathogenesis and Evolution Section, uses a cocktail of viruslike particles, or VLPs, of hemagglutinin proteins from four strains of H1, H3, H5 and H7 that have caused significant outbreaks in humans and animals. The VLP-based vaccine is administered in a two-step process where an initial vaccination primes the immune system and a second vaccine that boosts the immune response. It is being tested in ferrets after having success in mouse models.
This two-step approach is similar to that taken by the Israel-based company BiondVax Pharmaceuticals, which has a vaccine candidate in second-stage human trials in the U.S. and more advanced trials in Europe. The vaccine, called M-001 for now, works by first priming the immune system with peptides that span the length of hemagglutinin’s stems, followed up by a vaccine that uses a whole, heat-killed influenza virus to boost the response.
However, even if a vaccine candidate like M-001 were to become available in the next few years, it likely would be only the first major step in developing universal protection.
“Achieving an effective universal influenza vaccine will have to happen in an iterative way,” Graham said. “To start, we will try to make a vaccine that doesn’t have to be remade every year. And then a vaccine that hopefully wouldn’t have to be given every year.”
Old formulas, new tools
While the various universal vaccine candidates are put through their paces, researchers are working on novel ways to enhance vaccine efficacy by tweaking existing methods.

During a flu infection, the lungs mount their own immune response, which includes tissue-resident memory T and B cells as well as antibodies. In a paper in the journal Frontiers in Immunology, researchers at the University of Iowa led by Kevin Legge found that a nasally administered influenza A vaccine containing nanoparticles made up of biodegradable polyanhydride polymers could provide a robust immune response against both homologous and heterologous flu strains in the lungs of mice.
Legge and his colleagues wanted to generate the immunity that normally would be prompted by an infection without the symptoms and risk such an infection would carry. While standard vaccines already prompt an antibody and B-cell response, Legge said, years of work in animal models has suggested that the T cells play a significant role in controlling infection and also might give protection against heterologous challenges or flu strains with entirely different hemagglutinin proteins.
This further was corroborated when the pandemic H1N1 hit in 2009.
“Where researchers were looking at T-cell responses in humans … those patients that had T-cell responses did much better than those that did not,” Legge said.

When Legge’s group added the hydrophobic polyanhydride nanoparticles to the nasal spray vaccines, they found that the particles caused a stronger tissue-resident memory response. They were surprised when subsequent infection with proteins from an H3N2 virus was able to protect the mice from proteins from an H1N1 virus.
In addition to the nanoparticles and standard vaccine components, Legge and his colleagues had considered adding an anti-neuraminidase element to increase the vaccine’s efficacy. If the flu vaccines were able to confer protection against completely different flu strains, this would be another, albeit unanticipated, pathway to universal protection against flu viruses.
After working in the mouse model, which isn’t an ideal proxy for the human respiratory system, Legge and his colleagues tested the vaccine in ferrets, where it worked.
“It’s worked in two animal models, so we’re continuing to move forward to get this closer to being used in clinical trials,” Legge said.
Another method for tweaking existing vaccine production methods involves using a novel adjuvant that boosts vaccine strength such that the volume administered is small enough to be delivered by an intradermal, microneedle-dotted bandage. The system was described in September in the journal Science Advances by researchers at the University of Washington and the nonprofit Infectious Disease Research Institute in Seattle.

“Seventy to 80 percent of your immune system actually resides on the surface of your skin,” said Darrick Carter, the first author on the paper. “So when we give an intramuscular injection, we actually bypass a lot of these protective cells and inject right into the muscle where there are not that many immune cells.”
The researchers loaded up an available FDA-approved microneedle, the NanoPass Microneedle, with flu proteins grown recombinantly in and harvested from tobacco plants (see box: Strains, shots and sprays) and a novel adjuvant called GLA-AF, or aqueous formulation of glucopyranosyl lipid adjuvant. A different form of GLA previously had been demonstrated in intramuscular injections in humans to be able to reduce the standard dose of flu vaccine needed from 100 micrograms to 3.8 micrograms.
“Down the road,” Carter said, “what we want to do is to make this a self-administratable bandage where you would take it, remove a liner, put it on your skin, and then you’d be protected.”
If such a vaccine could be mailed to households, this could increase overall vaccine coverage by removing the need to visit a physician or pharmacist to become immunized, Carter said. As with all existing and developing flu vaccines, though, the greatest obstacle to a self-administered vaccine might be convincing people that they need to be protected from influenza in the first place.
Changing norms
Myths and misconceptions about the flu vaccine are pernicious and among the most significant reasons people decide against getting an annual flu shot.

According to Sandra C. Quinn, senior associate director of the University of Maryland’s Maryland Center for Health Equity and author of severalpapers about racial disparities in influenza vaccination rates in the U.S., interaction between patient and doctor is one of the most effective ways to build people’s trust in flu vaccines.
When Quinn and colleagues conducted interviews, focus groups and a national survey with black adults, they found that a physician’s recommendation had a significant effect on the likelihood that a black adult would get vaccinated.
“In our research, we see some things are on the side of the patient or people in the community and some things are on the healthcare system side,” Quinn said. “In (a recent) survey of 819 African-American adults, 25 percent said that the provider’s recommendation was fairly important, and 30 percent, extremely important.”
While black and white Americans both fall short of the goal of having 70 percent of the population immunized against flu, Quinn said, the disparity in protection is real. In the 2016-2017 flu season, the CDC estimates around 45 percent of white adults received a flu shot, while only 37 percent of black adults did. This carries ramifications beyond just exposure to flu, as the infection has additional risks for individuals with asthma, diabetes, heart disease, hypertension and obesity, all of which disproportionately affect black Americans.

“It’s really critical when people are in the doctor’s office, seeing the nurse practitioner, seeing the physician, that they get a strong recommendation, and particularly those people at high risk,” Quinn said.
While a universal flu vaccine is an undoubted boon, work will need to be done on an individual and community level to ensure that everyone who should get a vaccine does.
“This is not a case where ‘if you build it, they will come,’” Quinn said. “I think we’re going to have to do a lot of public education and provider education around vaccines and build trust in those vaccines as they come out.”
And over the past three decades, a wealth of epidemiologic studies have found that vaccinating schoolchildren against influenza can help protect high-risk members of society, including those who are immunocompromised or for whom the vaccine is less effective, such as the elderly. Mathematical modeling indicated that the same protective effect as immunizing 90 percent of adults above retirement age could be achieved by protecting 20 percent of children in primary and secondary school — a population that also hasn’t been overexposed to a lifetime of flu antigens.
“If we’re really going to be successful with a universal flu vaccine, we’re going to have to do it in children,” Fauci said. “We’re going to have to imprint the children with the universal flu vaccine, so that in any subsequent exposure they have to influenza, they’ll revert back to a response against the universal components.”
Antivirals

While flu vaccines were developed as early as 1938 and first distributed to U.S. soldiers in World War II, drugs that can reduce the duration of flu symptoms were not available until Tamiflu hit the market in 1999. The anti-viral, whose efficacy has been questioned in recent years, is recommended to be taken within 48 hours of the onset of flu symptoms and works by preventing neuraminidase proteins from cleaving sialic acids on the inside of a host cell. This leaves a virus functionally unable to exit the cells.
For the nearly two decades that Tamiflu has been available, the only other anti-virals approved by the FDA for treating influenza were also neuraminidase inhibitors. Relenza, a powder taken through oral inhalation, was approved in 2006; Rapivab, an intravenous option for treating swine flu, was approved in 2014, after seeing limited use during the 2009 pandemic following an emergency use authorization. A new product produced by the Japanese pharmaceutical company Shionogi, which uses a different mechanism to interfere with the protein’s activity, may upend that dominance, as it was recently approved by the FDA for distribution in the U.S., for which Shionogi will partner with the pharmaceutical company Roche.”
When faced with neuraminidase inhibitors, however, flu viruses sometimes can mutate to subvert the mechanism. At National Jewish Health, an academic medical research facility in Denver, Dennis Voelker is working with lipids to develop chemical inhibitors of viral replication that would preclude this by targeting an essential mechanism that the virus can’t mutate its way around.
“Our approach has been to look for inhibitors of viral attachment to cells,” Voelker said. He and his colleagues began working on viral inhibitors after finding that a phospholipid they’d been working with was able to bind to sialic acid receptors on the outside of flu particles essential to the virus’ ability to adhere to cells.
“If you mutate the ability to bind to phospholipids, you’re probably also going to mutate the ability of the virus to bind to the cell surface,” Voelker said. “What we really need is crystal structures showing just how the lipids interact with the virus, and we’re in the process of doing that.”
This box was updated on October 24 to reflect the FDA’s approval of Xofluza, and differs from the print version of the story.

Strains, shots and sprays
Each season’s flu vaccine protects against three or four strains of the virus, which are selected by experts convened by the World Health Organization every February for the Northern Hemisphere’s flu season and in September for the Southern Hemisphere’s flu season. These experts base their decisions on which flu strains have been found most prevalent by hundreds of surveillance laboratories around the world. While a number of factors are in play when the flu peaks in each hemisphere, including increased travel and children returning to school, one of the strongest drivers is the drop in humidity with colder temperatures, which is favorable to flu particle transmission, although the crowding in cities recently was found to make it easier for viruses to find new hosts in any weather.
For more than 70 years, viruses for these vaccines have been grown in hens’ eggs over several weeks, inactivated with heat and mixed with an adjuvant to stimulate the body’s immune response and a handful of preservatives at biologically safe levels, including formaldehyde and thimerosal. The vaccines then are administered with a microneedle, and over the next two weeks, recipients’ bodies begin producing antibodies against the three or four strains of virus of which the vaccine contains components. Both the universal vaccines developed at Mount Sinai that are now in clinical trials are prepared using this egg-based method.
In 2012, the FDA approved a faster, cell-based process that uses mammalian cells rather than hens’ eggs, to incubate flu viruses. The process is initiated with viruses that were grown in eggs. It takes days rather than weeks to generate enough flu antigens in the cells to produce vaccines. Additionally, the cells can be frozen, or “banked,” allowing for rapid vaccine production in the event of a pandemic event, without the need for using eggs. As of 2016, the vaccine, sold as Flucelvax, has been approved by the FDA to include four strains of flu virus.
In 2013, the FDA approved an entirely egg-free vaccine production method in which flu antigens are grown recombinantly in insect cells. In this process, manufacturers isolate hemagglutinin proteins from the targeted influenza viruses, combine them with a secondary virus that can grow in the insect cells and incubate the recombinant virus in the cells. After several days, the hemagglutinin proteins can be purified from the insect cells and incorporated into a vaccine. Additionally, older adults can get higher-dose flu shots or shots with an additional adjuvant, both of which induce a stronger immune response.
In 2018, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices reinstated its recommendation for a quadrivalent nasal spray vaccine, FluMist, which uses a live-attenuated virus grown with an egg-based process. The nasal spray, made by the pharmaceutical company MedImmune, first received FDA approval in 2003 but was not available for the past two flu seasons after the advisory committee recommended against using it due to lower than expected effectiveness. This season, MedImmune has been slower to roll out the vaccine than its competitors, possibly due to a delayed start in production.
When a nasal spray vaccine is administered, the upper nasal passages are exposed to a live but weakened virus that can’t replicate lower in the respiratory tract, which induces a more robust immune response in children than the standard inactivated flu vaccine administered intramuscularly via microneedle. The discrepancy in immune response is a consequence of viral exposure; when you breathe in a sneezy stranger’s airborne flu particles, they’re going to hit your nasal passages first and begin replicating there. If left unchecked, the viruses can work their way down into the lungs, where they can devastate bronchial tissue, create an opportunity for secondary bacterial infections or spur a cytokine storm, an all-out immune attack by the body that can wreak havoc on the lungs.

Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

Exploring lipid metabolism: A journey through time and innovation
Recent lipid metabolism research has unveiled critical insights into lipid–protein interactions, offering potential therapeutic targets for metabolic and neurodegenerative diseases. Check out the latest in lipid science at the ASBMB annual meeting.



