A solution that holds water
Name a biological function, and proteins called integrins are probably involved in it. Together, the 24 members of the integrin family allow cells to attach to one another and to the matrix that surrounds them. They help cells decide what to become, where to go, how to respond to their environments, and when to grow, divide or die.
Integrins’ ubiquity and versatility also mean that when cells bearing them go awry, these proteins can contribute to a range of diseases, from autoimmune diseases to cancer.
The FDA has so far approved six drugs that reduce the activity of specific integrins to treat illnesses such as multiple sclerosis and ulcerative colitis and to prevent blood clots from forming. To the disappointment of scientists, doctors and patients, however, other promising candidates have failed in clinical trials and curtailed integrins’ potential as treatment targets.
New work led by researchers at Harvard Medical School and Boston Children’s Hospital uncovers a reason for the failures — and offers a potential solution.
Taking a close look at an integrin involved in blood clotting, Timothy Springer at HMS and Boston Children's and colleagues found that failed drugs for two different integrins inadvertently encourage the integrins to open up into their “on” position, potentially driving integrin activity instead of quelling it.
The team revealed that in its closed or “off” position, the integrin contains a water molecule held in place by a series of chemical bonds. The integrin ejects the water molecule when activated.

Once they learned what was happening, the researchers were able to design integrin blockers that coaxed the clotting protein into its “off” position by holding the water molecule in place with a nitrogen atom.
Further tests hinted that water molecules play the same role in other integrins, indicating that the team’s strategy could work more broadly.
The findings, published in the journal Cell on Sept. 15, forge a clearer path for drug development and deepen researchers’ understanding of how integrins work normally.
“The same water-harnessing design principle has already been extended to another integrin, and structural information suggests that researchers can design drugs to target further members of the integrin family to treat diseases that cause great suffering,” said Springer.
“It’s always gratifying to work on a project that is both scientifically and medically important,” he added.
This article was republished with permission from Harvard Medical School. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
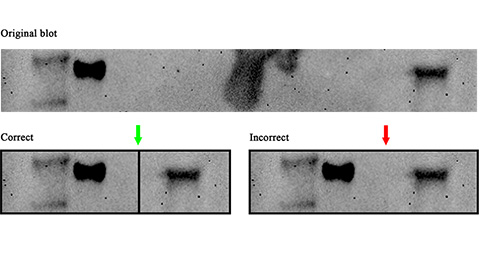
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.
Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

