UT Southwestern stem cell biologists develop embryo model
University of Texas Southwestern Medical Center biologists have developed a new stem cell-based embryo model for studying early human development, tissue formation and differentiation, offering valuable contributions to the field of developmental biology and regenerative medicine.
Peri-gastruloids are primitive embryonic structures with the potential to form many of the different cell types of the body, thereby providing a window into the earliest steps of embryogenesis. The findings on peri-gastruloids, published in the journal Cell, could impact a wide range of diseases and conditions that involve early human development, including developmental disorders, birth defects, genetic disorders and diseases that arise during embryonic development such as certain types of cancer or neurological disorders, researchers noted. Disease modeling and drug testing also can benefit from peri-gastruloid research.
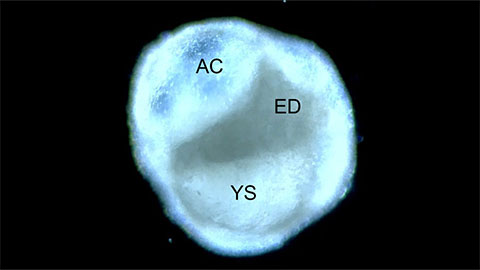
“By generating peri-gastruloids, researchers can mimic and study the complex processes that occur during gastrulation and early organ formation, which is a critical period in embryonic development. This allows for a better understanding of the molecular and cellular events that shape the formation of different tissue layers,” explained Jun Wu, assistant professor of molecular biology and member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern.
“Studying peri-gastruloids allows researchers to gain insights into how abnormalities or genetic mutations can impact the early stages of human development. This knowledge can be used to model and understand developmental disorders or diseases that arise during early embryonic development. Additionally, peri-gastruloids can serve as a platform for testing the efficacy and safety of potential drugs or therapies targeting these conditions,” Wu said.
Lizhong Liu was the lead author of the study. “The ability to model human development using peri-gastruloids can provide insights into the mechanisms behind tissue formation and differentiation. This knowledge can be applied to improve regenerative medicine approaches, such as generating specific cell types or tissues for transplantation or repairing damaged organs,” said Liu, an assistant instructor of molecular biology in the Wu lab, which focuses on using stem cell models to learn more about mammalian development and create regenerative medical applications.
While in vitro stem cell models that recapitulate human gastrulation have been established, they lack the essential extraembryonic cells needed for embryonic development, morphogenesis, and patterning. The new approach provides a robust and efficient method that prompts human pluripotent stem cells to self-organize into peri-gastruloids, which encompass both embryonic (epiblast) and extraembryonic (hypoblast) tissues. Although peri-gastruloids are not viable due to the exclusion of trophoblasts, they can simulate critical stages of human peri-gastrulation development, such as forming amniotic and yolk sac cavities, developing bilaminar and trilaminar embryonic discs, specifying primordial germ cells, initiating formation of primary germ layers, and early organogenesis.
By replicating conditions in vitro, researchers can delve into the underlying mechanisms and identify potential therapeutic targets for the eventual development of novel treatments or interventions for patients. Researchers also can expose peri-gastruloids to various drugs or compounds to evaluate their efficacy and potential side effects. This can help in identifying promising drug candidates and ensuring the safety of pharmaceutical interventions before they are tested in human clinical trials.
“Overall, the study’s findings on peri-gastruloids contribute to the advancement of clinical solutions by providing a platform for regenerative medicine, disease modeling, and drug testing, which have the potential to lead to the development of new therapies, improved treatment strategies, and enhanced safety assessment methods for the benefit of patients,” said Wu.
Generating new stem cells and stem cell-based embryo models to study embryogenesis and developing translational applications using stem cells represent the central directions of the Wu lab. The lab currently focuses on deriving novel pluripotent stem cells, identifying and overcoming the xenogeneic barriers, studying novel regulators of pluripotency, and generating stem cell-based models of mammalian embryos.
A version of this article was first published by the University of Texas Southwestern Medical Center. Read the original.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
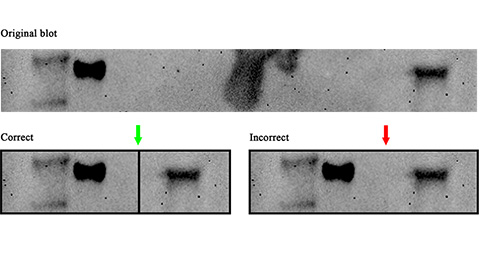
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.
Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

