When it comes to DNA replication, humans and baker’s yeast are more alike than different
Humans and baker’s yeast have more in common than meets the eye, including an important mechanism that helps ensure DNA is copied correctly, reports a pair of studies published in the journals Science and Proceedings of the National Academy of Sciences.

The findings visualize for the first time a molecular complex — called CTF18-RFC in humans and Ctf18-RFC in yeast — that loads a “clamp” onto DNA to keep parts of the replication machinery from falling off the DNA strand.
It is the latest discovery from longtime collaborators Huilin Li of Van Andel Institute and Michael O’Donnell of the Rockefeller University to shed light on the intricate mechanisms that enable the faithful passage of genetic information from generation to generation of cells.
“The accurate copying of DNA is fundamental to the propagation of life,” Li said. “Our findings add key pieces to the puzzle of DNA replication and could improve understanding of DNA replication-related health conditions.”
DNA replication is a tightly controlled process that copies the genetic code, allowing its instructions to be conveyed from one generation of cells to the next. In diseases like cancer, these mechanisms can fail, leading to uncontrolled or faulty replication with devastating consequences.
To date, at least 40 diseases, including many cancers and rare disorders, have been linked to problems with DNA replication.
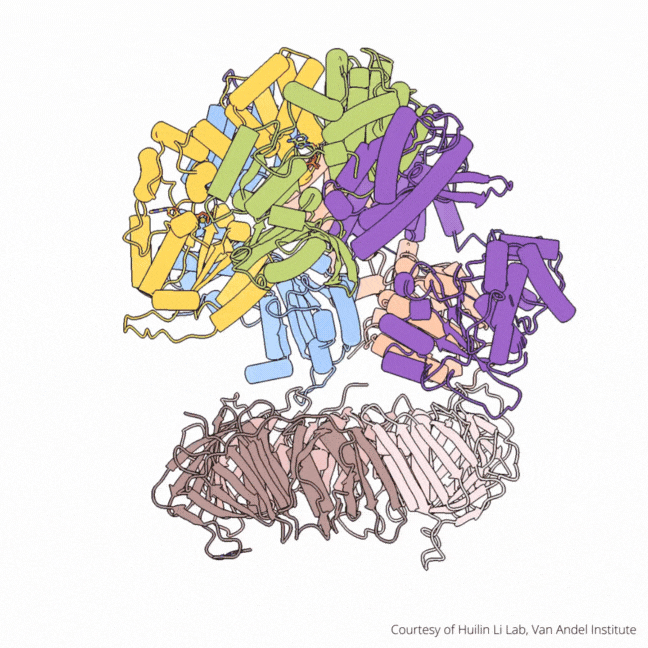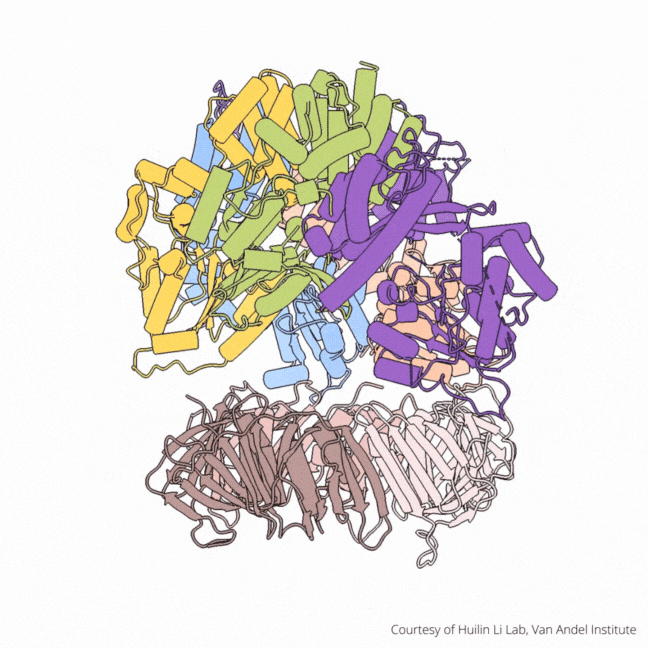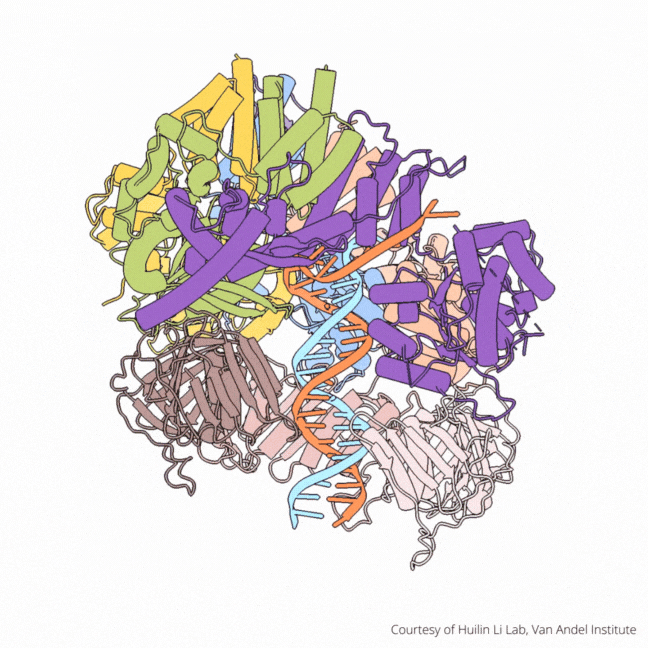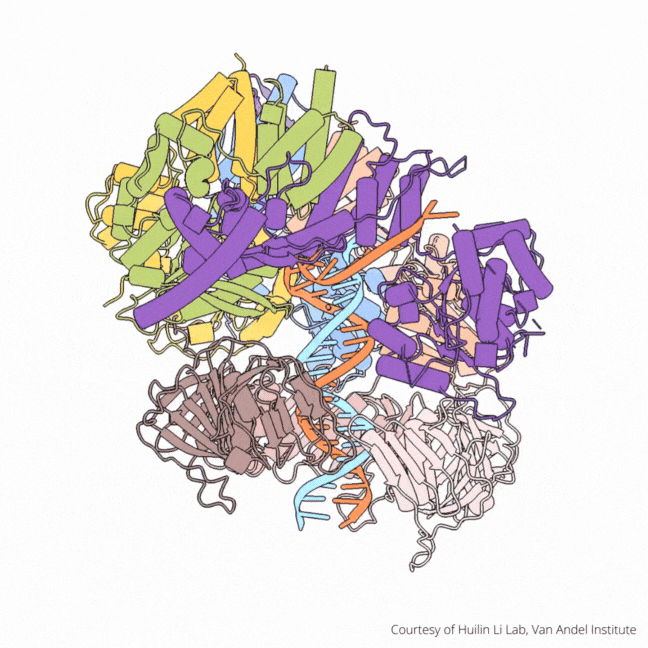
The process begins by unzipping DNA’s ladder-like structure, resulting in two strands called the leading and lagging strands. A molecular construction crew then assembles the missing halves of the strands, turning a single DNA helix into two. Much of this work falls to enzymes called polymerases, which assemble the building blocks of DNA.
On their own, however, polymerases aren’t good at staying on the DNA strand. They require CTF18-RFC in humans and Ctf18-RFC in yeast to thread a ring-shaped clamp onto the DNA leading strand, and another clamp loader called RFC in both human and yeast to thread the clamp onto the lagging strand. The clamp then closes and signals to the polymerases that they can begin replicating DNA.
Using high-powered cryo-electron microscopes, Li, O’Donnell and their teams revealed previously unknown facets of the leading strand clamp loaders’ structures, including a “hook” that forces the leading strand polymerase to let go of the new DNA strand so it can be recognized by the clamp loader. This distinction represents a key difference between the functions of the leading strand clamp loader (CTF18-RFC) and the lagging strand clamp loader (RFC) and illuminates an important aspect of varying DNA duplication mechanisms on the leading and lagging strands.
Lastly, the study identified shared features between the yeast and human leading strand clamp loaders, which demonstrate an evolutionary link between the two. This finding underscores the value of yeast as powerful yet simple models for studying genetics.
This article is republished from the Van Andel Institute website. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

Exploring lipid metabolism: A journey through time and innovation
Recent lipid metabolism research has unveiled critical insights into lipid–protein interactions, offering potential therapeutic targets for metabolic and neurodegenerative diseases. Check out the latest in lipid science at the ASBMB annual meeting.

