Mapping out the protein path to hearing
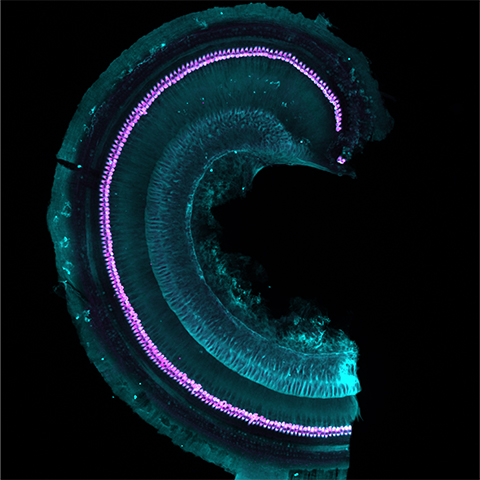
When sound-induced vibrations are translated into electrochemical signals, humans and other animals can hear those sounds. Within the cochlea of the inner ear, inner hair cells, or IHCs, release neurotransmitter-filled vesicles at their synapse with afferent neurons, sending auditory information to the brain. Some researchers suspect a unique network of trafficking proteins and organelles is involved in this neurotransmission. However, scientists do not know the identities for most of these molecular players.
Previous studies reported a general protein profile of IHCs by analyzing whole cochleas. However, these studies did not identify the proteins found specifically in the IHC’s presynaptic regions, where vesicles are released.
Andreia Cepeda, who studied deafness during her Ph.D. at the University Medical Center Goettingen, wanted to uncover these details.
“One of the challenges has been the scarcity of material needed for these kinds of studies which require large amounts of purified sample,” Cepeda said. “Obtaining enough starting material from these IHCs is an extremely difficult and time-consuming task.”
To tackle this challenge, Cepeda teamed up with Momchil Ninov, who specializes in isolating synaptic vesicles, and others from the Max Planck Institute. The group developed a protocol that led to the first complete map of the IHC proteome, which they published in their recent article in Molecular & Cellular Proteomics.
Before identifying IHC proteins, the team had to isolate enough samples from mice for proteomic analysis, Cepeda’s most challenging task.
“I was the only one dissecting the cochleas,” she said, “and I had entire weeks where I was dissecting every day, all day simply to collect enough material.”
Cepeda and Ninov said they isolated roughly 150 organs of Corti per run, the receptor organs where the IHCs are located, from these cochleas — which further reduced the amount of sample they had to work with.
“Imagine how many cochleas (and how many mice) we need to obtain enough material to be used for further purification,” Ninov said. “To give you an idea, one starts with the cochlea of a mouse, which is about the size of a grain of rice, and under the microscope, we need to dissect (the organ of Corti), which is the size of a needle’s head.”
Though tedious, this step notably improved their sample purity for downstream analysis.
Following dissection and homogenization, the group separated the samples into subcellular fractions by differential centrifugation. Then they immunoisolated IHC-specific vesicles and organelles using an antibody against VGluT3, a glutamate transporter expressed exclusively in IHCs. This resulted in an enriched, albeit limited amount of material ready to be characterized by mass spectrometry. The group had access to a sophisticated mass spectrometer allowing them to identify proteins in very small amounts.
Using this workflow, the team introduced the first exhaustive list of proteins involved in vesicle trafficking at the IHC presynapse. They also noted significant developmental changes in this molecular profile by measuring proteins before and after the onset of hearing. The group further validated the proteins by confocal microscopy but they say that more needs to be done. Knocking down these proteins in animal models along with recent advances in the study of membrane trafficking could reveal key synaptic physiology contributing to deafness.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

Redefining excellence to drive equity and innovation
Donita Brady will receive the ASBMB Ruth Kirschstein Award for Maximizing Access in Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Mining microbes for rare earth solutions
Joseph Cotruvo, Jr., will receive the ASBMB Mildred Cohn Young Investigator Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

