From the journals: JLR
How lipogenesis works in liver steatosis. Removing protein aggregates from stressed cells. Linking plasma lipid profiles to cardiovascular health. Read about papers on these topics recently published in the Journal of Lipid Research.
How lipogenesis works in liver steatosis
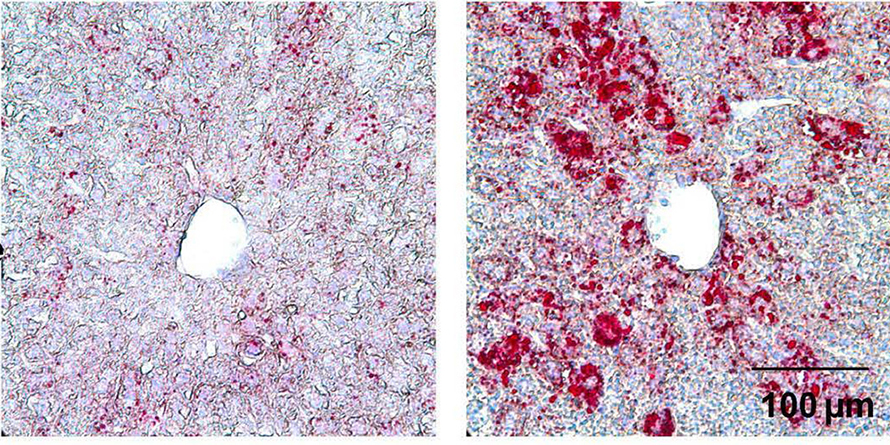
Fatty liver or hepatic steatosis affects over 3 million Americans every year, increasing the risk of disorders such as Type 2 diabetes, nonalcoholic fatty liver disease and obesity. Yunhong Huang and a research team from the City University of Hong Kong, China, recently uncovered the role of a transcription factor, KLF2, in mediating lipid metabolism and maintaining cholesterol homeostasis in the blood and liver. Although a previous study showed that reduction of KLF2 expression could have positive effects on liver steatosis, this new study published in the Journal of Lipid Research explored the role of KLF2 in promoting lipogenesis.
Using a liver-specific Klf2-overexpressing adenovirus, Huang and colleagues showed that overexpression of KLF2, both in culture conditions and in genetically altered mice increased fat retention promoting liver steatosis in mice. Using mice modified to overexpress KLF2, the researchers showed that this gene induces lipogenesis and underlies liver steatosis in mice fed a normal diet. Exploring the downstream signaling pathways, they showed that KLF2 promotes maturation of the master regulator SREBP1, which induces the expression of downstream genes involved in lipogenesis. Using chromatin immunoprecipitation–polymerase chain reaction analyses, the researchers showed that KLF2 regulates the protein SREBP1 by binding to the promoter region of the membrane protein SCAP. They also showed that reduced KLF2 downregulated the expression of SCAP- and SREBP1-associated target genes.
This study shows that KLF2 is involved in lipogenesis in the liver, leading to steatosis. The researchers also show that KLF2 is involved in maintaining blood cholesterol levels. This research provides a solid foundation for examining KLF2 as a therapeutic target to combat liver steatosis.
Removing protein aggregates from stressed cells
When a cell is stressed, protein aggregates known as “stress granules” form, and they must be removed to restore stability in the cell. Melanie Kovacs, Florian Geltinger and a group at the Paris–Lodron University in Austria recently showed the essential role of mitochondria in eliminating these stress granules. They published their work in the Journal of Lipid Research.
The researchers found that mitochondria and lipid droplets internalize these aggregates, particularly those tagged with the ATPase Ola1p, which these researchers call a “super aggregator.” They showed that this mitochondria–lipid droplet protein shuttling during stress can help detoxify the cell and keep it healthy.
The team observed that Ola1p-tagged protein-loaded stress granules moved from mitochondria to lipid droplets when a cell is stressed. This facilitates stress granule removal and can inhibit proteotoxic effects of these stress aggregates whose persistence can lead to neurodegenerative disorders. This movement is a useful backup strategy when other proteolytic processes to eliminate the granules fail.
This study developed and used proximity labeling and reporter–based co-localization studies to understand lipid droplet–protein aggregate relationships, which could be an excellent model for other aggregate dissolution studies. Understanding how cells manage stress could help researchers develop strategies for tackling diseases linked to protein build-up and open avenues to develop therapies for age-related diseases.
Linking plasma lipid profiles to cardiovascular health
Cardiovascular disease remains a top killer worldwide as scientists try to understand the genetic drivers of lipid abundance that increase this disease risk in humans. Using techniques such as ion mobility spectrometry and genetic linkage, a new study published in the Journal of Lipid Research mapped and identified the region of DNA affecting lipoprotein abundance and function from the plasma lipoprotein subfractions from 500 Diversity Outbred mice (genetically diverse mice used to identify genetic drivers of disease). Tara Price and colleagues at the University of Wisconsin–Madison cross-referenced these lipoprotein subclasses to the human genome to link mouse and human data, identifying genes that might drive lipid accumulation.
The study noted a gene encoding neutral ceramidase, Asah2, a novel candidate driver linked to large high-density lipoprotein particles known as HDL-2b, which are good predictors of human heart disease. To understand the role of Asah2, the researchers characterized mice that had been genetically altered to lack Asah2 and found that various lipoproteins in these mice were affected, as opposed to unaltered mice; specifically, HDL levels increased among mice lacking Asah2.
This method could be used to study other candidate genes, which might widen understanding of lipoprotein abundance and open avenues for treatment of cardiovascular diseases.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

