JBC: Researchers decipher how barbiturates work
Modern medicine would be unthinkable without the use of anesthesia during surgical procedures. In a recent paper in the Journal of Biological Chemistry that was selected as one of the Editors’ Picks, Marc Delarue of the Pasteur Institute in France and colleagues solved how a class of drugs called barbiturates function to induce anesthesia. “This work describes, for the first time, the three-dimensional structure by X-ray crystallography of barbiturates bound to the target receptor,” says Zaineb Fourati, one of the first authors on the paper. She adds, “Understanding the mechanism underlying anesthetics’ action is the first step toward the conception of better ones that are more specific and with less side effects.”
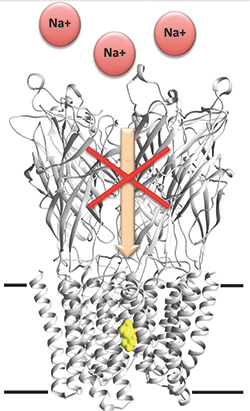 Researchers unveil barbiturates’ mechanism of action.IMAGE COURTESY OF MARC DELARUE
Researchers unveil barbiturates’ mechanism of action.IMAGE COURTESY OF MARC DELARUE
Barbiturates, a class of drugs derived from barbituric acid, made their debut in Germany in 1903. Their name is thought to be a fusion of “Barbara” and uric acid, a component of urine. Sources are divided on which female inspired the name, who could have been St. Barbara or someone less than a saint.
The first commercially available form of barbiturates was marketed as sleeping pills. Other medical applications of barbiturates soon emerged, including as anesthetics, sedatives and anticonvulsants, which led to their widespread use in the first half of the 20th century. However, the side effects of barbiturates led to their regulation and decreased popularity.
“Uncontrolled use of barbiturates could have hazardous consequences, from addiction to death by overdose,” says Fourati. “One concrete example is the presumably intentional death of the famous Marilyn Monroe due to a barbiturate overdose in the early 1960s.” Nevertheless, barbiturates remain essential in the treatment of epilepsy and as an intravenous general anesthetic.
Anesthetics act by decreasing the relay of information in the nervous system. Specifically, anesthetics affect several types of membrane-embedded ion channels. The principal targets of barbiturates are thought to be anionic and cationic pentameric ligand-gated ion channels, known as pLGICs. Although there is some evidence to show that barbiturates bind to the cylindrical pore of the ion channel, the exact nature of this interaction is unclear.
To understand this interaction better, Delarue and colleagues used the bacterial pH-gated receptor GLIC, which has high structural similarity to mammalian pLGICs. They first showed that the bacterial version of the channel behaved much like its eukaryotic counterpart by demonstrating that a specific barbiturate called pentobarbital can inhibit the electrical current of the bacterial GLIC channel.
Next, the investigators used three barbiturate derivatives — bromobarbital, thiopental and selenocyanobarbital — that were modified chemically such that they displayed an unusual electron scattering behavior to help assign bound ligands in the X-ray structure unambiguously. The investigators showed that all three barbiturate derivatives bound to the closed GLIC channel deep within the central pore, and they used computational simulations of the channel in open and desensitized states to support their findings further.
“This work partly reveals how barbiturates induce anesthesia,” says Fourati. “As these drugs act on the nervous system, it is very important to address the exact mechanism of their action, and the structure provides a direct proof of a molecule binding to a given site.”
She and her colleagues think that the knowledge gained in this study can help design a new generation of barbiturates not only for anesthesia but also for psychiatric and neurological disorders.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
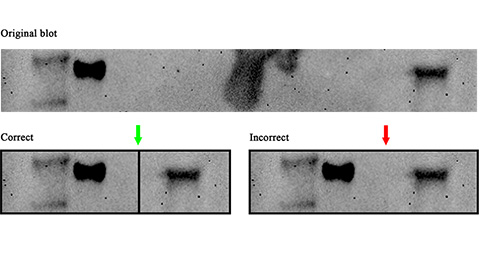
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.
Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

