Neurodegenerative disease linked to microtubules
First characterized in Quebec in 1978, autosomal recessive spastic ataxia of Charlevoix–Saguenay, or ARSACS, is a hereditary neurodegenerative disease. Symptoms such as difficulty walking often appear in early childhood and continue to progress, limiting the mobility and lifespan of those affected.
In particular, ARSACS affects the cerebellum, the region of the brain that controls motor skills. It is the second most common recessive form of ataxia, or loss of muscle coordination and movement, in the world.
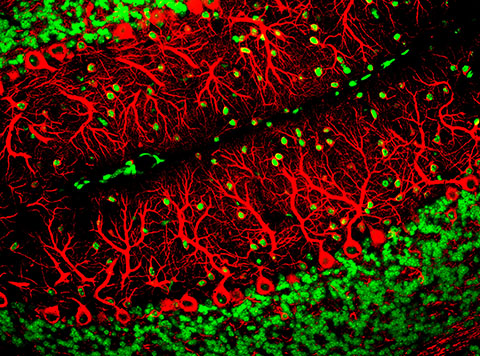
No cure exists for ARSACS, but in 2000, a team at McGill University identified mutations in the protein sacsin as its cause. Developing therapeutics is a challenge, however, because researchers do not completely understand sacsin’s function. Although previously published work suggests sacsin may influence mitochondrial transport and function in neurons, its role in the cell is still unclear.
Vincent Francis, a postdoctoral fellow at McGill University, joined the laboratory of Peter McPherson because he was interested in neurodegeneration. In particular, Francis wanted to work on the understudied sacsin.
“I decided to pursue the project to understand the cellular function of sacsin, which could provide potential new therapeutic strategies for the treatment of the disease,” Francis wrote to ASBMB Today.
Previous work in the lab had focused on mitochondria, so Francis began looking at the transport of other organelles. He focused on the lysosome, the recycling center of the cell, where unwanted materials can be broken down and reused. Generally, lysosomes are clustered neatly around the nucleus. However, in cells without sacsin, lysosomes were scattered all around.
Lysosomes and other organelles are transported on microtubules. In neurons without sacsin, lysosomes move less. Based on their observations, Francis and the team hypothesized that sacsin could regulate the trafficking of cargo on microtubules.
“We assumed that sacsin could probably be functioning as an adaptor for organellar transport,” Francis wrote. “Instead, what surprised us was the ability of sacsin to bind to microtubules and to modulate microtubule dynamics.”
Microtubules are required for autolysomal reformation, a process in which new lysosomes are formed. Once again, without sacsin, cells showed a decrease in this process.
Because neurons are large, expansive cells, regulation of organelle trafficking is particularly important for their function.
This research, recently published in the Journal of Biological Chemistry, suggests sacsin is a key regulator of cellular traffic. In the future, the team hopes these results will inform research that can help identify treatments for patients with ARSACS.
Francis noted that several other neurological disorders — including Alzheimer’s disease — are associated with decreases in neuronal microtubule stability. This indicates that microtubules may be a promising therapeutic target for ARSACS and other neurodegenerative diseases.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

Exploring lipid metabolism: A journey through time and innovation
Recent lipid metabolism research has unveiled critical insights into lipid–protein interactions, offering potential therapeutic targets for metabolic and neurodegenerative diseases. Check out the latest in lipid science at the ASBMB annual meeting.

