‘A good ambassador’
Hudson Freeze almost didn’t return the phone call he received to tell him he was one of the recipients of the 2013 Golden Goose award. “I got it as a recorded message from one of the people involved,” he says. “I was thinking, ‘Oh Lord, this is the prize patrol. I’ll have to sign up for 15 different journals for two years, and they are going to give me some sort of prize for doing that,’” he says, laughing. “But I decided to call them back.”

 The Golden Goose award is given to scientists and engineers who have done federally funded research projects that appeared to be odd or insignificant at first but went on to have sizable impacts on science and society. Freeze won the award along with his undergraduate research adviser at Indiana University, Thomas Brock, for a discovery that has changed how molecular biology research is done.
The Golden Goose award is given to scientists and engineers who have done federally funded research projects that appeared to be odd or insignificant at first but went on to have sizable impacts on science and society. Freeze won the award along with his undergraduate research adviser at Indiana University, Thomas Brock, for a discovery that has changed how molecular biology research is done.
Freeze and Brock were the first to isolate Thermus aquaticus, which they recovered in samples they collected at Yellowstone National Park’s hot springs during the summer of 1966. Thermostable Taq polymerase, on which the polymerase chain reaction is based, was later isolated from this bacterium.
The undergraduate project was a sign of Freeze’s ability to do science that makes an impact. Over the course of his career, Freeze, now based at the Sanford-Burnham Medical Research Institute in La Jolla, Calif., has focused on glycosylation. “He’s the world leader in the study of congenital diseases of glycosylation,” says Gerald Hart of Johns Hopkins University. “What’s really unique about Freeze’s work is he’s a hardcore basic scientist, but he applies his work to human diseases and actually treats children” with those diseases. Freeze’s laboratory now does whole exome sequencing to identify as many genes as possible in patients with congenital glycosylation disorders. (Freeze says that, at his last count, there were 99 distinct congenital glycosylation diseases.)
So how does a person go from isolating a thermophilic bacterium to studying glycosylation disorders? It seems it starts with a curiosity about extreme and odd life forms.
“Lit a fire under me”
Freeze grew up in Garrett, Ind., with his parents and younger sister, Jackie. “Dad had graduated from high school probably near the bottom of his class, and Mom never graduated from high school,” says Freeze. “But they were both bright, and they understood the importance of education.”
Freeze’s father worked as a brakeman and conductor on the Baltimore & Ohio railroad system, which was founded by John. W. Garrett, after whom their town was named. His mother stayed home to take care of Jackie, who is mentally disabled.
The high school Freeze attended had only 500 students. It was there that Freeze caught the science bug from Jack Bateman, who taught chemistry and biology. “He just lit a fire under me,” says Freeze. “I remember the first day of biology class. He said, ‘People, I’m going to take you on a very exciting trip. We are going to learn things, and believe me, you’re going to work hard. But we are going to learn things that you will not even believe. You will not believe how interesting biology is.’”
Under Bateman’s guidance, Freeze got hooked on astrobiology and won a science fair with his project about possible life forms on Mars. The interest carried him to major in microbiology at Indiana University. After his sophomore year of college, Freeze went to see Brock about a research assistant position. Brock asked him if he would like to accompany him to Yellowstone National Park to look for microorganisms in the hot springs. Freeze was thrilled to be asked. His high-school interest in astrobiology was still there, and the hot springs represented an extreme environment that promised to have interesting critters.
On that trip in August 1966, Freeze and Brock collected water samples from various hot springs over several days. Brock’s cabin at the national park had a room turned into a laboratory where they prepped the collected samples and got them ready to be taken back to Indiana University. During the fall semester of his junior year, Freeze got down to the business of growing, isolating and characterizing the bacteria.
Initially, Freeze almost gave up on Taq. The first tubes of it he tried to grow had a dilute medium. Over a few days, there wasn’t much turbidity, a sign of multiplying bacteria, in the tubes. At last, he spotted something at the bottom of one test tube: They were salt crystals. Freeze was disappointed, but he continued to let the tubes incubate in the hot bath. “A day or two later, I picked up another tube and looked at that. I said, ‘Oh, more crystals.’”
But this time, Freeze decided to stick a few crystals under a microscope. “As soon as I got it under the microscope, there were these long strings of bacteria. It was the absolute thrill of discovery at that point because” — here, his voice drops to a stage whisper — “I was the first person in the world to see these things!”
Freeze isolated Taq, put it in another medium, and experimented with its growth conditions. The description of Taq was his first scientific publication, which he coauthored with Brock.
Going off script
 |
| A young Hudson Freeze. |
The fun with Taq spurred Freeze to continue on to graduate school at University of California, San Diego, in 1969. When he got there, Freeze fell in love with another peculiar organism called Dictyostelium discoideum. Its life cycle captured his imagination. Known in everyday parlance as slime mold, the organism transforms from a collection of unicellular amoebae into a multicellular slug.
Under William Loomis’ guidance, Freeze decided, for his Ph.D. thesis, to figure out how the surface sheath around the slime mold slug formed. He says, “It turned out that there were carbohydrates in there. I didn’t know anything about carbohydrates.”
Fortunately, another graduate student was knowledgeable about sugar analysis by gas chromatography, so Freeze teamed up with him. As Freeze’s research progressed, he says, “What became important is that there were a number of different kinds of mutants that you could isolate in Dictyostelium.”
Some of the mutants didn’t make the surface sheath. Curious, Freeze began to investigate the enzymes involved in putting together the sheath. He eventually tracked down some mutations in lysosomal enzymes. Freeze says it wasn’t clear how those mutations would affect a structure like the surface sheath, but he did note that there were abnormalities in some glycoproteins.
As Freeze was untangling how the slime mold put its sheath together for his Ph.D. thesis, he was also moving around in the Los Angeles entertainment business. “Clearly I wasn’t a star and ended up as a scientist instead,” he says self-deprecatingly.
Freeze had acted in high school plays and had decided to join a small acting group that did dramatic readings at independent coffeehouses. “This is in the early 1970s. Tom Waits was one time with us. This is when Tom Waits was completely unknown,” says Freeze. “He was this weird guy who sat over by the piano, all hunched over.”
Freeze caught the attention of a director looking to cast someone in the role of Brick, the lead male role in Tennessee Williams’ “Cat on a Hot Tin Roof.” Freeze balked at first, thinking he wasn’t good enough. But then he agreed. As the show ran, the director introduced Freeze to a Los Angeles acting coach named Sal Dano. Dano told Freeze he saw potential in him as a mainstream actor, provided that he lost some weight and got himself an agent. Dano then invited Freeze to join the master acting classes he taught once a week in San Diego. Freeze was bowled over by Dano’s confidence in him. “I was thinking, ‘Get an agent! Oh my god! This guy is talking some serious stuff!’” recalls Freeze. “I got this boost that I never thought was possible. So, yeah, I joined Sal’s class. I got pictures taken. I got an agent.”
Freeze won a national acting talent search launched by Paramount Studios. He did commercials for JC Penney and the Chevrolet Corvette and modeled clothes. From those gigs, “I was making as much money from acting as I was in graduate school. But of course, that was only $2,000 a year at that time, not a big thing,” he says. Freeze landed the lead role in an independent industrial film called “Forests for the People,” which, when Freeze last checked, can be found only in the Maureen and Mike Mansfield Library at the University of Montana. “I’m sure it’s protected by armed guards day and night,” he quips.
But he soon had to make a decision on what to pursue, and the decision was relatively easy to make. “I missed science,” he says. “I realized how stupid a lot of this stuff was that was going on in the [acting] business. I thought, ‘I can do something better than this.’”
From slime mold to sick kids
As Freeze continued on at UCSD as a postdoctoral fellow between 1976 and 1979, he kept at the problem of the mutated glycoproteins in the slime mold surface sheath. He eventually worked out that the glycoproteins were missing mannose-6-phosphate. Mannose-6-phosphate gave Freeze his first insight into clinical research.
There is a human disorder called inclusion-cell disease, “which was mysterious back in the late 1970s,” says Freeze. Around the time Freeze discovered that slime mold had mistakes in mannose-6-phosphate processing on some lysosomal enzymes, I-cell researchers also realized that the enzymes they were working with involved mannose-6-phosphate. Freeze spent a year at Washington University in St. Louis in the laboratory of Stuart Kornfeld, which focused on I-cell disease. “The environment at Wash U and in his lab was just mind-boggling. This lab worked around the clock. You had people on the day shift and on the night shift,” says Freeze. “It was so exciting there. They were getting at some of the first biosyntheses of carbohydrates that were defective because of gene mutations.”
Freeze made an impression in the Kornfeld laboratory. “Hud is a Midwestern boy,” says Ajit Varki at University of California, San Diego, who had introduced Freeze to Kornfeld. “The day he was supposed to arrive in St. Louis, there was a blinding snowstorm. Everything was shut down. But he came driving through from San Diego in a Honda Civic, all by himself, without any trouble. We were so surprised to see him show up on that day!”
As Freeze worked in the Kornfeld laboratory, it became clearer that the work he was doing in slime mold could improve our understanding of human diseases. And the realization spurred a conundrum: Should he go to medical school to be better equipped to do clinical work or continue doing fundamental research? When he returned to San Diego, he decided to apply for both medical school and a grant from the National Institutes of Health. “Believe it or not, both of those things came through in the same week,” says Freeze, still sounding surprised after all these years. But after some soul-searching, Freeze decided to stay in research, because he felt it would keep him challenged in the long run. Nonetheless, as he continued to work with slime mold glycosylation, he kept his eye out for any human disorders that could benefit from his work.
The link between research in an amoeba and human disorders came in the mid-1990s. Varki had received some cells from a clinician. These cells had been taken from children who appeared to have glycosylation disorders. Freeze, collaborating with Varki, began to analyze the cells. “I did the same experiments on those cells that I would have done on my slime mold cells in terms of labeling sugar chains with radioactive mannose,” says Freeze. “When I did that, I saw some of the human cells actually displaying the same kind of abnormalities that I saw in the slime mold. I said, ‘Oh my God, this is the right kind of connection. There is something worthwhile here.’”
Human glycosylation defects were largely unexplored territory at the time, and, because of that, Freeze found himself in an interesting position: “In the mid-1990s, there wasn’t very much knowledge of glycobiology. You’d have to walk the country far and wide to find a glycobiologist. Physicians would say, ‘Based on these little tests, I can see my patient has some glycosylation disorder, but none of my doctor friends knows anything about this. Where do I go?’ They were forced to engage with basic scientists like me because of the vacuum that was there.”
Over the past 17 years that Freeze has worked with congenital glycosylation disorders, he has met many patients. His office and laboratory walls are plastered with photos of children with these diseases. Freeze says growing up with his disabled sister has made him very comfortable around disabled children.
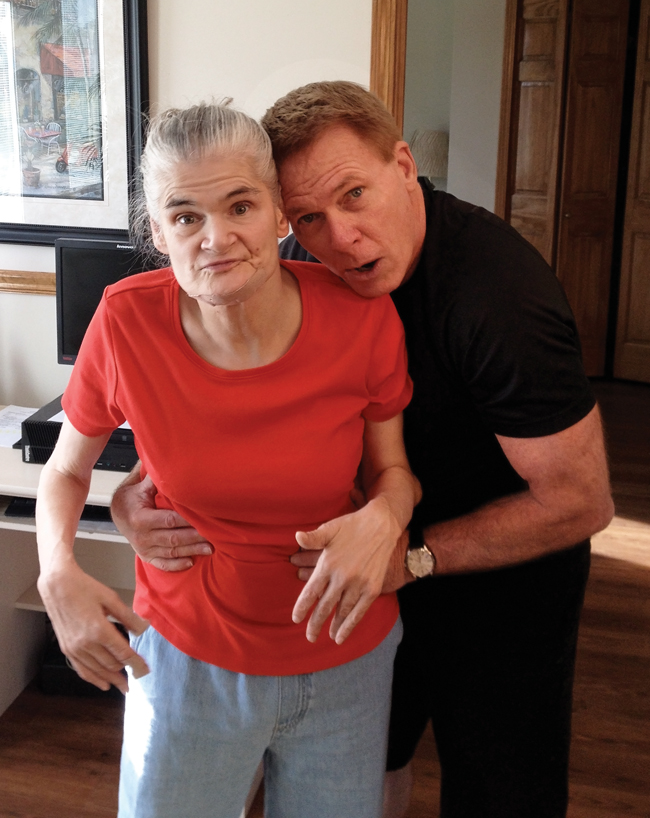 |
| Hudson Freeze and his sister. |
The boy in gray lederhosen
There is one little boy who stands out the most in Freeze’s mind. In the mid-1990s, Freeze had a couple of German medical students do fellowships in his laboratory. Their supervisor in Germany, Thorsten Marquardt, noticed a paper in which Freeze’s group had demonstrated that they could correct a particular glycosylation defect in cells by simply adding mannose to the cell culture medium. Marquardt called Freeze to tell him that he was caring for a 6-year-old boy who had an unknown glycosylation defect, but the defect probably was different from those in the cells Freeze’s group had studied. The boy was in an intensive-care unit in Munich with unstoppable gastrointestinal bleeding. He was close to dying.
‘“We will do anything. Do you have any idea how much mannose you might give him?’” Freeze remembers Marquardt asking him.
Just two days before, Freeze and his colleagues had finished the calculations on some data. Freeze had received permission from the U.S. Food and Drug Administration to test mannose as a potential drug. He and several of his lab members spent two weekends holed up in the conference room, drinking solutions of mannose and then measuring its concentration in their blood over the course of the day to understand its pharmacokinetics. Based on the numbers they had crunched, Freeze was able to tell Marquardt how much mannose solution to give to the child over a period of time. But he told Marquardt he had no idea if the treatment would work, wished him luck and hung up the phone.
Over the next eight months, as the boy’s doctor gave him solutions of mannose based on Freeze’s calculations, the boy got better. The gastrointestinal bleeding stopped within the first few weeks, and his chronic diarrhea came to an end. Freeze was incredulous when he heard the news. He was certain that the mannose was a red herring and something else had reversed the boy’s symptoms.
On his next trip to Europe, Freeze stopped off in Munich to meet the boy’s doctor as well as the boy and his mother. “This kid shows up in gray lederhosen. He was the cutest thing you ever saw,” says Freeze. “The doc was very protective of him, and he showed me the improvement of clinical symptoms after the boy was on mannose. We said, ‘Yeah, that is improvement!’”
But they still didn’t know why the mannose treatment was working. The null experiment would be to stop giving the child mannose and see what happened, but that experiment would be unethical. “We went down to the beer garden, and the doc said, ‘Look, I’m keeping him on mannose. You do whatever you need to do, but we’re keeping him on mannose,’” says Freeze. Then Freeze, Marquardt and the two German students who had spent a stint in Freeze’s laboratory began to muse over beers what could be happening in the boy.
Freeze had received some of the boy’s cells months earlier so that his team could analyze them. Before he had left on his trip, they had noticed that the boy’s cells incorporated more radioactive mannose on the glycoproteins than the control cells when the cells were fed radioactive mannose by cell culture. That was an important clue.
Normally, mannose-6-phosphate is derived from glucose through a series of steps. One of these steps is the action of phosphomannose isomerase on fructose-6-phosphate to make mannose-6-phosphate. (The enzyme can work in reverse and convert mannose-6-phosphate into fructose-6-phosphate.) Mannose-6-phosphate eventually winds up in glycoproteins.
In the beer garden, Freeze wondered out loud if the boy had a mutation in phosphomannose isomerase that meant his body couldn’t make mannose-6-phosphate from fructose-6-phosphate.
“Well, I was on vacation,” says Freeze. The two medical students who already had returned to Germany from their stint in Freeze’s lab stayed up day and night running assays on the boy’s cells. When he returned to San Diego, “they called me at the equivalent of midnight their time. They said, ‘We got it. You were right. It was phosphomannose isomerase.’”
The boy had a mutation that made phosphomannose isomerase work inefficiently. “Now it all made sense,” says Freeze. The mannose treatment was overcoming the inability of phosphomannose isomerase to make mannose-6-phosphate. The mannose fed to the boy was being converted into mannose-6-phosphate by other enzymes and allowing the N-glycosylation of necessary proteins to proceed. The boy’s particular disorder is now known as congenital glycosylation disorder Ib, or CDG-Ib for short. The boy has since grown into a young man.
Varki notes this success story has a dark underlining. “Unfortunately that kind of work was taken advantage of (by) charlatans who sell large quantities of sugars on the web, saying that healthy people need seven essential sugars and all that nonsense,” he says. “Hud and Ron Schnaar, who was president of the Society for Glycobiology, actually took the trouble to go on ABC News’ ‘20/20’ once and talk about this problem. It is really damaging our field.”
Varki says that Freeze has done all he can to shut down those fraudulent outfits, who are armed to the teeth with lawyers. He adds the fact that Freeze takes the trouble to try to rein in unwelcome consequences of his work shows “he has a sense of commitment that goes beyond just science.” Varki also points out that outreach and advocacy for science are important to Freeze; he is the vice president-elect for science policy at the Federation of American Societies for Experimental Biology (the American Society for Biochemistry and Molecular Biology is a member organization). “He’s the perfect person for it, because he’s very articulate and thoughtful,” says Varki. “He’s a good ambassador for science.”
Freeze says he still uses what he learned from his acting days to give scientific lectures and engage audiences when doing science outreach. Varki and Hart report that Freeze is known to pull out a guitar and perform rock songs at conferences. When he’s not traveling, Freeze spends an evening every week as a tenor in a black gospel choir (“I’m the white pixel in the choir picture,” he notes). He loves gospel because “it has so much heart and soul.” He pauses briefly and then says, “Because you want to give, emotionally, everything you can.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

McRose awarded Packard fellowship
She will receive $875,000 in research funding over five years.

Redefining excellence to drive equity and innovation
Donita Brady will receive the ASBMB Ruth Kirschstein Award for Maximizing Access in Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

ASBMB names 2026 fellows
The American Society for Biochemistry and Molecular Biology announced that it has named 16 members as 2026 fellows of the society.

ASBMB members receive ASM awards
Jennifer Doudna, Michael Ibba and Kim Orth were recognized by the American Society for Microbiology for their achievements in leadership, education and research.

Mining microbes for rare earth solutions
Joseph Cotruvo, Jr., will receive the ASBMB Mildred Cohn Young Investigator Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

McKnight wins Lasker Award
He was honored at a gala in September and received a $250,000 honorarium.

