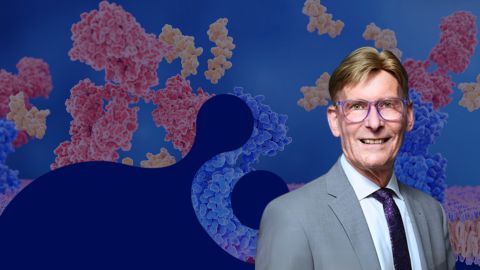
Meet Philip Cole
Philip Cole first encountered the Journal of Biological Chemistry in an airport. Then a Yale University undergraduate, he was flying to Maryland to visit Johns Hopkins University. The M.D./Ph.D. program director had promised to meet Cole at the airport with a telltale prop: “a JBC under his arm” — the latest issue of the journal.
Decades later, from 2016 to 2022, Cole would serve on the JBC editorial board. He joined the ranks of JBC associate editors in May 2022.

Cole earned a bachelor’s degree in science at Yale University, then a postgraduate certificate in chemistry as a Churchill Scholar at England’s University of Cambridge. He completed a medical degree and a doctorate in bioorganic chemistry at Johns Hopkins University School of Medicine. Cole did clinical and postdoctoral training at Harvard Medical School and Brigham and Women's Hospital, then a stint at Rockefeller University as a junior lab head.
In 1999, Cole joined the Johns Hopkins faculty as a professor and director of pharmacology until 2017, when he returned to Boston. He has served for about six years as a professor of medicine, biological chemistry and molecular pharmacology at Harvard Medical School and has been interim chief of the Division of Genetics at Brigham and Women’s Hospital since early 2022.
Cole and his lab study the chemical biology of protein post-translational modifications, or PTMs, with an eye to signaling, epigenetics and cancer. The team devises and uses such chemical methods as protein semisynthesis and small-molecule probes to explore protein phosphorylation, acetylation, ubiquitination and other PTMs in cell networks and enzymes.
In addition to his work for JBC, Cole has reviewed studies for 29 research journals, notably Science, Nature, Cell and the New England Journal of Medicine.
Cole spoke with Paula Amann, ASBMB Today’s science writer, about his research career and his history with JBC. The interview has been edited for clarity and length.
How did your early research set you on your path in biochemistry?
My Ph.D. project concerned an enzyme called aromatase, which converts androgens to estrogens. At that time, there was a lot of enthusiasm for developing inhibitors of aromatase for the treatment of breast cancer.
I was trying to understand the chemical mechanism of aromatase, which involves a series of hydroxylations and then an aromatization step catalyzed by the cytochrome P450 enzyme. That project was really influential in making me think about complex enzymatic transformations, how enzymes catalyze difficult reactions and how one could develop innovative approaches to study such complicated reactions.
I also immersed myself in steroid hormones and endocrinology and learned about their fascinating metabolic pathways, their various effects on tissues and the pharmacology related to steroid transformations and therapeutics. Learning about hormones laid the foundation for my future training in endocrinology at Brigham and Women's Hospital.
As a postdoc, you worked with Christopher T. Walsh. What drew you to his lab, and what did you take away?
I chose to work with Chris Walsh because he was one of the leaders of mechanistic enzymology at the time. He was also located at an institution, the Harvard Medical School, where I wanted to train both clinically and in research. When I met him, he was a larger-than-life figure. I remember spending several hours with him during my transition from my M.D./Ph.D. training to residency training, and he was extremely welcoming and encouraging.
I'd actually met him two or three years before when I was still a student at Hopkins and he was a visiting lecturer. He told us about this new department of biological chemistry and molecular pharmacology that he was founding at Harvard.
The Walsh lab was populated by an amazingly strong series of trainees, mostly postdoctoral fellows from all over the world, very accomplished people. They covered a lot of different areas of science, from hardcore chemistry to sophisticated biochemistry and genetics. It was an atmosphere filled with energy, ideas and collegiality. Chris really set the tone: encouraging people to interact with each other and to work together, to share problem-solving and learn from each other.
Chris just passed away in January, which has been a profoundly sad thing for myself and many, many of his trainees in the field.

You've published a number of papers in the JBC, starting early in your career. What did that mean for you?
My impression of the Journal of Biological Chemistry was formed when I first heard of the journal as an undergraduate.
I was applying for M.D./Ph.D. training, and one of the institutions I was interested in was Johns Hopkins. The program director at Hopkins, who was on the JBC editorial board, said he would pick me up from the airport. This was quite a nice gesture, considering I was just an undergraduate and he was a senior faculty member at Johns Hopkins. He said I’d be able to spot him as I walked off the plane because he’d be the guy with a JBC under his arm.
I didn't know what a JBC was, but I asked around. I was an undergraduate at Yale. and I remember asking some people in my little circle there. We deduced that it was probably the Journal of Biological Chemistry. I saw that he had this big blue book under his arm. That's how we connected, and that was my first introduction to the JBC.
When I did ultimately join Johns Hopkins for training, I learned that the department I was working in, pharmacology, was founded by a towering figure in biomedical science named John Jacob Abel. Among many achievements, he was the founding editor, along with Christian Herder, of the JBC in the early 1900s.
The Journal of Biological Chemistry had a very important place in the history of Johns Hopkins and the pharmacology department and became a destination journal for the best science you can publish. I was fortunate to publish two papers in JBC, as a postdoc in Chris Walsh's lab, on tyrosine kinase mechanisms.
I was really proud of these two papers, one of them related to the kinetic mechanism of tyrosine kinases, and the other on their chemical mechanism. These were papers that helped to shape my thinking about how these kinases could be studied and what the challenges were in thinking through their mechanisms. It really helped to lay the foundation for my future lab.
On the strength of those two JBC papers, I was able to get a faculty position at Rockefeller, so those papers were clearly pivotal for me.
Your group has designed kinase and other enzyme inhibitors. What's happening with those, and what are the potential applications?
Our lab has had a long-standing interest in developing inhibitors using mostly design approaches — as opposed to screening for the identification of enzyme inhibitors and targeting enzymes that are potential therapeutic targets. These enzymes, including kinases, have over the last 20 years been clearly demonstrated to be important, pharmacologic targets in medicine.
Our work in the area of kinases has been less about leading directly to therapeutics and more about understanding the structure regulation and substrate selectivity of protein kinases. The kinase area quickly became populated by large pharma after we started working on our early-stage compounds. We felt that trying to develop our own kinase inhibitors in our small academic lab would be superseded by the amazing talent and resources brought to bear by many pharmaceutical companies.

We shifted over to histone-modifying enzymes. We've worked on two main classes of targets: the histone acetyltransferase area, and particularly a pair of enzymes called p300 and CBP, CREB-binding protein. These efforts ultimately led to high-quality small molecule inhibitors and the founding of a small biotech company called Acylin Therapeutics Inc, which improved upon our initial work and led to candidate compounds for clinical development. The compounds and this technology were acquired by the major pharmaceutical company, AbbVie.
A second, recent area of work has concerned targeting histone deacetylase enzymes. In particular, we’re making compounds that can target specific histone deacetylase complexes. One of our compounds, called corin, shows promise in a number of cancer applications.
How did you come to join the JBC editorial board and then serve as an associate editor?
As far as becoming a member of the editorial board, I remember jumping at the chance. I had been an editor for other journals, but I had such a profound respect for the JBC that it was something that I embraced.
The JBC has an excellent and deserved reputation for rigor and novelty. I consider it elite without being elitist, meaning that it's very high quality and among the best science that is published, but it doesn't have a certain quota of papers that it can publish. It allows for all sorts of very high-quality science to be published.
I think that's what we strive for. I also liked the fact that it's affiliated with the American Society for Biochemistry and Molecular Biology, and the society supports really important initiatives in science, communication and training—helping both academic and industry labs.
Of all the projects underway in the Cole lab, what is most intriguing to you?
I’m very excited about the work we’re doing on designer chromosomes to try to understand how modifications on the histones can influence the structure of the nucleosome, chromatin and its binding partners. These dynamic interactions and changes lead to increased exposure of DNA and increased gene expression, and govern things like cell proliferation, differentiation of cells and disease processes involving gene expression.
Chromatin is composed of building blocks called nucleosomes. Each has approximately 147 base pairs that have double-stranded DNA that wraps around eight proteins, an octamer of core histone proteins. The nucleosome becomes the architecture around which our chromatin is based.
Designer nucleosomes involve modifying histones so that they include specific post-translational modifications and using a range of techniques to introduce modifications in the nucleosomes. These modifications include lysine acetylation, lysine methylation, lysine ubiquitination, serine phosphorylation and arginine methylation.
We have been developing strategies to prepare chromosomes with specific modifications. We’re trying to determine whether there’s cross talk between chemical modifications on nucleosomes, the building blocks of chromosomes. It’s a very vibrant area of research: figuring out if one modification on a nucleosome can affect what’s going on with another. We believe that the designer nucleosomes that we make can be employed to help us understand how to best target epigenetic enzymes to treat cancer and other diseases.
Research can be a long slog. How do you stay motivated when progress is elusive?
It takes stick-to-it-ive-ness and grace in the face of challenge. My siblings, my parents and my kids — one’s a medical student and the other is pursuing show business — have modeled these qualities.
When my daughter was starting high school, she wanted to participate in a computer app competition with a group of girls, but this activity did not exist at our local school. She helped create a branch of a computer science club that received regional recognition and went on to be highly successful for years after she funded it.
Talking to and learning from many different kinds of people in and out of the lab can also help. For example, our research team had been trying to trap a nucleosome bound to an enzyme, Sirt6. This trapping can be really helpful to obtain a high-resolution structure of the nucleosome-enzyme complex. Unfortunately, the strategy we thought would work was not panning out. Then my postdoc read other studies, especially a key one from a lab in China led by another former postdoc of mine, and figured out a new trapping approach that worked.
Given your many interests, what’s kept you engaged with our society?
The ASBMB has touched on many of the topics that I consider most near to my scientific heart: biochemical studies on enzymes, gene expression and molecular biology. Rigor and the emphasis on mechanism: That is embodied by the ASBMB.
The society meetings that I attend have always been highlights for me. Now, my trainees both attend and present there. I can network with all sorts of wonderful colleagues that I rarely get to see otherwise. These are really valuable experiences.
Being in the ASBMB has also been about professionalism. We're focusing on how scientists should treat each other, and how they should share the highest ethical standards in their work. In recent years, I've appreciated its focus on diversity, equity and inclusion—the need to recognize historical marginalization that's happened in the scientific community and efforts to remedy that through important initiatives.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Embrace your neurodivergence and flourish in college
This guide offers practical advice on setting yourself up for success — learn how to leverage campus resources, work with professors and embrace your strengths.

Survival tools for a neurodivergent brain in academia
Working in academia is hard, and being neurodivergent makes it harder. Here are a few tools that may help, from a Ph.D. student with ADHD.

Quieting the static: Building inclusive STEM classrooms
Christin Monroe, an assistant professor of chemistry at Landmark College, offers practical tips to help educators make their classrooms more accessible to neurodivergent scientists.

Hidden strengths of an autistic scientist
Navigating the world of scientific research as an autistic scientist comes with unique challenges —microaggressions, communication hurdles and the constant pressure to conform to social norms, postbaccalaureate student Taylor Stolberg writes.
Richard Silverman to speak at ASBMB 2025
Richard Silverman and Melissa Moore are the featured speakers at the ASBMB annual meeting to be held April 12-15 in Chicago.

Women’s History Month: Educating and inspiring generations
Through early classroom experiences, undergraduate education and advanced research training, women leaders are shaping a more inclusive and supportive scientific community.

