Breaking through limits in kinase inhibition
Protein kinases, enzymes that add phosphate groups to other proteins, are often dysregulated in diseases. This makes kinase inhibitors popular drugs, although they often target things they aren’t supposed to. To mitigate these off-target effects, scientists like Paul Shapiro are finding ways to target specific functions of a kinase, rather than inhibiting the whole enzyme.

Shapiro was the first speaker on ASBMB Breakthroughs, a new webinar series highlighting research from ASBMB journals. He is a professor in the department of pharmaceutical sciences at the University of Maryland School of Pharmacy and associate editor at the Journal of Biological Chemistry, or JBC. In his talk, sponsored by JBC, Shapiro discussed taking ideas and discoveries from basic science research toward clinical applications.
The most common types of kinase inhibitors, known as Types 1 and 2, compete with ATP binding and lock kinases in either an active or inactive conformation, respectively. On the other hand, Type 3 kinase inhibitors are not ATP competitive. They prevent enzymatic reactions by binding to a catalytic site adjacent to the ATP pocket.
Shapiro works with what he calls Type 4 kinase “modulators.” Instead of targeting the ATP binding or catalytic sites, these modulators target regions involved in a kinase’s interaction with its substrates.
“We call them modulators because they don’t inhibit all enzyme activities,” Shapiro said.
He explained how kinases often have both a “pro” and “anti” function. For example, a kinase can be “pro” proliferation or inflammation, but also modulate these “pro” functions through “anti”, or negative feedback.
“Type 4 modulators allow us to selectively target signaling functions that we want to inhibit while preserving other potentially beneficial functions,” he said. “Think of it as targeting the gas and keeping the brake.”
Shapiro’s work focuses on mitogen-activated protein kinases, or MAPKs, specifically p38α and extracellular signal-regulated kinases, or ERKs. MAPKs have regions known as D- and F-recruitment sites, or DRS and FRS, that recognize D- and F-sites on substrates, respectively. Based on what researchers knew about how MAPKs recognize substrates, Shapiro teamed up with Alex MacKerell to use computer-aided drug design to identify compounds that have shown efficacy in pre-clinical studies and have advanced to clinical testing.
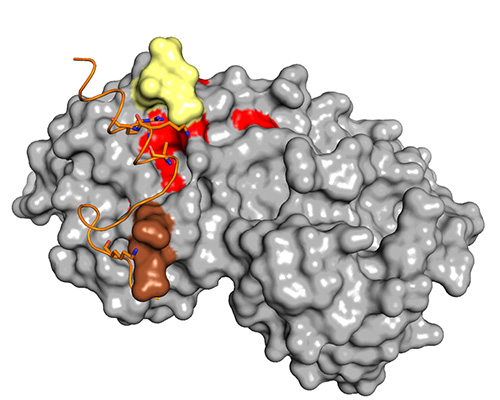
Shapiro and his collaborator, David Wiest at the Fox Chase Cancer Center, study ERK substrate specificity in myeloproliferative disorders, a type of blood cancer in which the bone marrow produces too many blood cells. They found that D-site substrates of ERK are more involved in disease progression and F-site substrates more in disease attenuation. Shapiro, Wiest and their colleagues showed that compounds that specifically target ERK’s DRS can mitigate overactive proliferation in myeloproliferative disorders.
“This is a unique example of how ERK can have pro and anti functions depending on its docking site,” Shapiro said.
In another project, Shapiro works with Deepak Deshpande at Thomas Jefferson University. Their team developed a compound that targets a region near ERK’s FRS and showed it can reduce airway remodeling, a process in asthma that leads to thickened airway walls and narrowed airways, in mice treated with dust mites to produce symptoms mimicking human asthma.
Shapiro also collaborates with Jeffrey D. Hasday, a University of Maryland colleague, on a compound that targets the inflammatory role of p38α in acute respiratory distress syndrome, or ARDS.
“p38 inhibitors have been around for 30 years,” Shapiro said. “But none of them have made it into the clinic because most of them target both the α and β isoforms.”
p38α is more involved in pro-inflammatory functions, while p38β typically has a more protective role in cells. In addition to this difference, even the p38α isoform alone has both “gas” and “brake” functions.
“The goal was to primarily target the gas functions,” said Shapiro. “In p38α, that involved substrates that utilize the DRS.”
The modulator Shapiro’s team developed, called UM101, targeted p38α’s pro-inflammatory function, sparing the β form and preserving p38α’s “brake.” Researchers recently reported an optimized version of UM101; it is currently in a clinical trial testing its safety and efficacy in human ARDS patients.

Up next
Lipidomics, shotgun lipidomics and functional lipidomics for Alzheimer’s researchFeb. 12, 2025 12:15–1 p.m. Eastern
Xianlin Han of the University of Texas Health Science Center will present his research on the use of shotgun lipidomics in biological and biomedical contexts.
Register for the whole ASBMB Breakthroughs webinar series
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
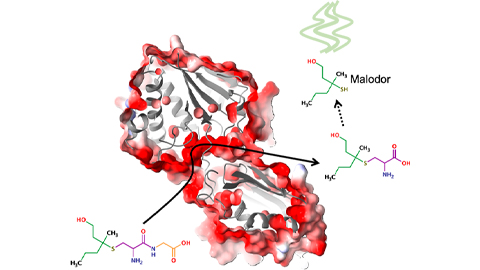
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.
Proteomic variation in heart tissues
By tracking protein changes in stem cell–derived heart cells, researchers from Cedars-Sinai uncovered surprising diversity — including a potential new cell type — that could reshape how we study and treat heart disease.

Parsing plant pigment pathways
Erich Grotewold of Michigan State University, an ASBMB Breakthroughs speaker, discusses his work on the genetic regulation of flavonoid biosynthesis.

