With curiosity, a career is both a journey and a circle
Of all the honors and affiliations a scientist might collect over a successful career, being featured on the IMDb — the Internet Movie Database for you uninitiated folks — has to be among the rarest.
The IMDb website is where you learn about your favorite movie actor or director or discover whether the distinctive voice on your kid’s favorite animated series really is Patrick Stewart. (Answer: If it sounds like the former Starship captain, it probably is.)

John Peters, ASBMB member and co-recipient of the 2020 National Academy of Science’s Cozzarelli Prize and the 2023 Faraday Horizon Prize from the Royal Society of Chemistry, is also an IMDb-credited producer of a PBS documentary film: “Search for the Origin of Life.” And he’s cited for an on-screen turn in the role of “scientist.”
The documentary was produced around the time I got to know Peters as my new colleague when I first arrived at Montana State University. He was a faculty member and the director of MSU’s NASA Astrobiology Center. His work at the center and with exotic microbes reflected a longtime interest in deep questions about life, its origins, and its potential to survive in unlikely places.
Peters’ lab in the chemistry and biochemistry department was adjacent to mine, and he became something of an informal mentor. I remember one piece of excellent advice that countered the conventional wisdom of university administrators, especially during those financially lean times. He said: Always stretch your horizons scientifically, but don’t waste time writing grants you don’t think you can get. Instead, take the time to build quality projects you believe in, write good papers, and let the results speak for themselves.I would see him both practice and preach this advice as a scientific collaborator and coauthor.
A winding road
Peters’ career trajectory has had its share of twists and turns. In a professional climate where linearity is less and less the norm, up-and-coming scientists might take comfort. A career punctuated by change and a more-than-occasional shot of risk can lead you to someplace where you might enjoy the view.
In Peters’ case, that includes some distinguished numbers: a paper count somewhere north of 180 and an H-index (56) that could be an admirable resting heart rate but is also equivalent to the median for National Academy of Sciences inductees. He has been a faculty member at four institutions and is now chair of the department at his undergraduate alma mater, the University of Oklahoma. He has three accomplished children living in far-flung parts of the U.S., who he regularly checks in on, often taking his Sprinter van.
I recently asked Peters about the journey that took him from his college days in Oklahoma and back there again.
“I was really unfocused as a student and just interested in everything,” he said. “As an undergraduate, I had majors like geology, then changed to engineering. I liked writing a lot, so I thought I was going to be studying English, but that didn't really work. … I think I was just really curious,so I just kept changing my major … and then I took a couple of microbiology classes.”
The microbiology department at Oklahoma had some of the few U.S.-based faculty investigating anaerobic metabolism. The subject seemed to Peters both out on the fringes and utterly fundamental. They were asking deep questions about life and the boundary conditions for survival: How, in principle, could an organism get the necessary building materials and energy to function, especially without the powerful driving force provided by the reduction of oxygen?
This line of questioning would lead Peters to other strongholds of anaerobic microbiology: Virginia Tech, and then CalTech.
“When I finished my Ph.D. at Virginia Tech, structure was the thing that was going to solve all the problems,” he said, “so I wanted to go to a structure lab that was open to the type of problems that I was interested in, like anaerobic metabolism. Doug (Rees)'s lab was about the only one out there. He collaborated with Mike Adams on all these weird enzymes that were in hyperthermophiles. A lot of those were anaerobes. And then, he was working on nitrogenase like I'd been working on as a grad student.”
A huge feat
When it came time to begin his own research program, Peters aimed high, looking for a challenging problem where structural work would be impactful. In the so-called iron-only hydrogenase, he found a perfect match. A review of hydrogen biochemistry by Dick Holmes had thrown down the gauntlet: The iron-containing hydrogenase was the subject of much speculation but no structural data.
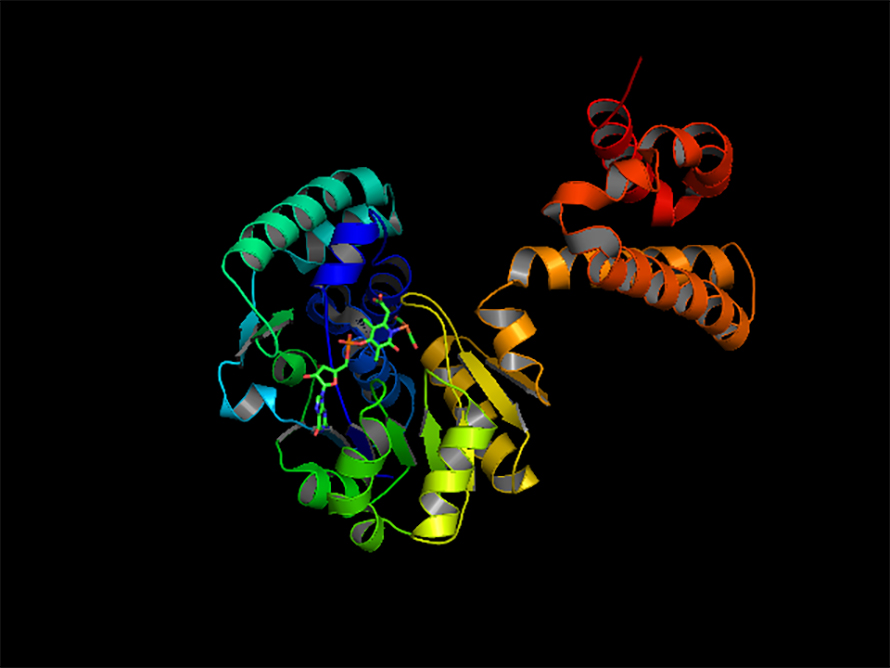
“It was an interesting time to do structures where we didn’t really have everything figured out,” Peters said. The database contained far fewer structures to use as models, and the technology for working anaerobically or under cold temperatures was not well developed.
At the synchrotron, he set up and carried out a complex data collection experiment basically on his own. The beam line didn’t even have a simple detector. “I measured the whole thing on film at that time,” Peters recalled, “and then when I saw I had gotten really good data, I was just amazed. I was almost sick to my stomach. … I was thinking when I was looking at it that. … I'm the first person to ever see this thing. This is just amazing, and it's unique.”
Solving the structure of a large, multiple-metal, air-sensitive enzyme was a huge technical feat. But when I asked him what he thought was significant about that period in his career, Peters said: “What makes me proud of that achievement is that it spun off a lot of unexpected projects and sustained a bunch of other people's careers … based on ideas that those structures were able to put into motion.”
Good science grows good science. The structural biology of hydrogenases opened a series of important questions. How do these oversized enzymes bring together two tiny electrons and two protons, catalyzing their combination to make hydrogen gas? How are the reactions catalyzed by a nickel-iron alloy, iron and metal-free hydrogenases alike? What biases the reactions in one direction or other? Why are some hydrogenases more and some less oxygen-sensitive? How are their metalloclusters (and models of them) built? How did these catalysts evolve and diversify? What controls the biological interplay between hydrogen gas and reactions that make or consume methane, either in the same organisms or in different organisms within the same community? And last but not least, what are humanity’s prospects of learning from some of nature’s tiniest creatures about how we might make and use hydrogen gas as a fuel, safely and efficiently?
Uniting diverse themes
Curiosity had driven Peters toward multiple potential undergraduate majors, and it’s led his lab’s published work toward diverse research themes. Early experiences with the technical challenges of hydrogenases were, in some respects, liberating; they freed him to take on some thorny but significant questions in the anaerobic arena. His group’s work on the structural biology of nitrogen fixation, for example, has evolved to encompass the regulatory proteins that control the pathway. They’ve shown that other pathways, once identified in relatively restricted metabolic contexts, are in fact much more broadly distributed. They described, for example, the production of methane-evolving coenzyme M outside of methanogens and systems for fixing carbon dioxide beyond pathways involving phototropism or organic acid metabolism.

Uniting all this work is a fundamental, career-long interest in biological electron transfer to and from hydrogen–hydrogen, nitrogen–hydrogen and carbon–hydrogen bonds. The parent metabolic pathways involved — hydrogen production and consumption, nitrogen fixation, methanogenesis — are fundamental to global element cycling and biological energy generation.
Most recently, Peters’ group embarked on an investigation of microbe-driven electron transfer in one of its most elegant and technologically compelling forms. In electron bifurcation, pairs of electrons enter an enzyme with equivalent potential energy but proceed to their destinations with driving forces that are no longer the same. This process results in one electron effectively donating some of its driving force to the other.
To understand bifurcation, a researcher be able to organize the scaffold of proteins involved and all the electron-transferring cofactors they depend on. And then, they must imagine the whole protein machine acting as a sort of thermodynamic set of chutes and ladders for electrons, moving them from cofactor to cofactor until they reach their endpoint. It’s the kind of scientific puzzle that demands broad expertise and seems built for teamwork. Peters’ bifurcation team was recognized with the Cozzarelli Prize in Physical and Mathematical Sciences.
The word “curiosity” comes up frequently when talking to Peters about what drives his career. When employed strategically, curiosity is more than fascination. It’s an instinct that tells us when something merits deeper attention.
Back in the early days, when Peters and I co-taught a seminar course in astrobiology, he invited a speaker from NASA’s Curiosity rover project. That mission was motivated by a simple question: Did Mars ever have the right environmental conditions to support microbes, and could it do so today? Curiosity landed more than a decade ago with an expected two- to three-year life-span. Like Peters’ work, it continues to explore the possible origins and limits of life in anaerobic environments to this day.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreFeatured jobs
from the ASBMB career center
Get the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Embrace your neurodivergence and flourish in college
This guide offers practical advice on setting yourself up for success — learn how to leverage campus resources, work with professors and embrace your strengths.

Survival tools for a neurodivergent brain in academia
Working in academia is hard, and being neurodivergent makes it harder. Here are a few tools that may help, from a Ph.D. student with ADHD.

Quieting the static: Building inclusive STEM classrooms
Christin Monroe, an assistant professor of chemistry at Landmark College, offers practical tips to help educators make their classrooms more accessible to neurodivergent scientists.

Hidden strengths of an autistic scientist
Navigating the world of scientific research as an autistic scientist comes with unique challenges —microaggressions, communication hurdles and the constant pressure to conform to social norms, postbaccalaureate student Taylor Stolberg writes.
Richard Silverman to speak at ASBMB 2025
Richard Silverman and Melissa Moore are the featured speakers at the ASBMB annual meeting to be held April 12-15 in Chicago.

Women’s History Month: Educating and inspiring generations
Through early classroom experiences, undergraduate education and advanced research training, women leaders are shaping a more inclusive and supportive scientific community.

