RNA binding proteins with benefits
Relationships formed between proteins seem far from romantic — unless you’re a biochemist.
The diverse and genetically complex environment of the cell is home to a multitude of proteins with varying functions. Unlike the character-driven romances of the big screen, interactions among proteins can be unpredictable, are largely transactional and are sequence- and structure-dependent.

And promiscuity isn’t necessarily a bad thing.
In protein biology, the term promiscuity describes proteins that are capable of a wide swath of interactions within the cell, with other proteins, small or large molecules and other substrates. Enzymes, or reaction-driving proteins are labeled promiscuous when they can catalyze multiple reactions.
Proteins in a subcategory that execute the same reaction with varying substrates are called substrate-promiscuous, and when substrate-promiscuous enzymes make unique products from these reactions, they are labeled catalytically promiscuous.
Evolutionary biologists have largely described promiscuous protein activity as not physiologically relevant because these interactions are inefficient in producing biological outcomes. In the last two decades, however, research interest has shifted toward promiscuous proteins, particularly a new enthusiasm for understanding their potential evolutionary advantages and exploiting the biological flexibility of promiscuous proteins for drug discovery.
Blanton Tolbert, a professor of biochemistry and biophysics at the University of Pennsylvania, studies the biochemical mechanisms of RNA virus replication, with the goal of identifying novel targets for therapeutic intervention. Tolbert’s lab focuses on characterizing the heterogeneous nuclear ribonucleoprotein A1, or HNRNPA1, a notoriously promiscuous RNA-binding protein that is involved in cutting and folding, or splicing, RNA, in addition to RNA metabolism, transport and processing.
“I have always been interested in molecular recognition,” Tolbert said.
What began as childhood curiosity and wanting to understand nature and the natural world evolved into “a career where I actively investigate protein–RNA interactions that drive physiological outcomes at the biochemical and biophysical level,” he said.
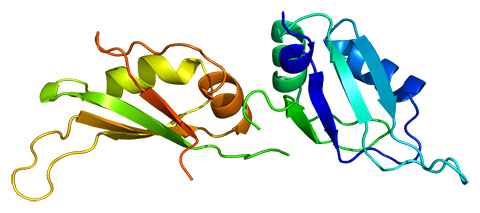
A shape shifter
More than a decade of continued effort by Tolbert and his team to understand HNRNPA1 recently culminated in a paper in the journal Science Advances. Tolbert’s group, in collaboration with Iowa State University, Ohio State University and Washington University School of Medicine, created a conceptual model for HNRNPA1 that depicts the way the RNA-binding protein regulates multiple biological processes using shape-changing mechanisms. The big discovery of this research, however, is what underlies the shape-changing mechanism of HNRNPA1.
“The protein appears to be promiscuous,” Tolbert said, “but we find the promiscuity is actually a fundamental feature of the protein.”
The researchers found that the thermodynamic coupling of the two domains of HNRNPA1 is so strong that RNA binding to one domain is directly communicated to the other, and, if disrupted, the RNA-dependent protein interaction and subsequent functions are obstructed.
“The way HNRNPA1 binds RNA targets is context-dependent,” Tolbert said. “This means the RNA as a substrate will change how it binds and assembles (to HNRNPA1) to drive specific functional outcomes.”
Tolbert’s lab has also successfully collaborated on developing compounds that can prevent the replication of enterovirus A-71, which can cause polio-like symptoms, and the COVID-19 virus, SARS-CoV-2.
“The way that we think about science is portable to other systems because, at the end of the day, it is about the questions we ask at the molecular level,” Tolbert said. “We were able to pivot from HIV to enterovirus to SARS- COV-2 and then to influenza because the principles of molecular recognition are agnostic to the virus.”

Inclusion that benefits everyone
Tolbert was inspired to run an academic lab and mentor students by his experience during graduate school, specifically his cultural isolation as the only black American in his Ph.D. cohort.
“I wanted to immerse myself in science,” Tolbert said. “Year after year, I’m listening to different speakers, and none of them looked like me. That’s when I made a conscious decision to run an academic research lab.”
Tolbert also raised two children while pursuing higher education.
“Throughout my entire academic career, I have had a family — I raised two wonderful young men,” he said. “Being engaged and involved in their life fed back into my work and how I approached it.”
Tolbert continues to be an advocate for all students. Before moving to UPenn in 2023, he served at Case Western Reserve University as the inaugural vice dean of diversity, equity and inclusive excellence, and as the Case Comprehensive Cancer Center’s first associate director for diversity, equity and inclusion. In this role, he designed initiatives to recruit individuals from backgrounds underrepresented in science for tenure-track positions that would influence, engage and benefit students from all walks of life.
“When speaking to colleagues about inclusion, I often say, ‘We have a periodic table of elements, we have many different cell types, various molecules — we study and are intrigued by the diversity and heterogeneity of the natural world,’” Tolbert said. “’Something happens when we shift from talking about biological systems to people, cultures, communities and society, where diversity is approached with hesitation or aversion.’”
In 2022, Tolbert became the vice president of science leadership and culture at the Howard Hughes Medical Institute, where he oversees a variety of programs dedicated to improving the resources for upcoming scientists, in addition to developing strategic inclusion-centered initiatives. His focus at HHMI is to make science more accessible, and more interesting, for all people.
“If we are thinking about the individuals who are on the margins of STEM when we design these initiatives, then we benefit everyone,” he said.
While his role at HHMI requires Tolbert’s full attention, he makes a point to be in the lab with his students at least once a week, where he has cultivated a culture of altruism, inclusion and thirst for knowledge.
“I want to understand what drives the individuals I mentor, what they bring from their lived experience, and how I can meet them where they are,” he said.
Tolbert has no entry requirements or gatekeeping when he takes on new students in his lab. He understands and stresses the importance of providing opportunities and creating attainable paths for successful student outcomes.

Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in People
People highlights or most popular articles

Embrace your neurodivergence and flourish in college
This guide offers practical advice on setting yourself up for success — learn how to leverage campus resources, work with professors and embrace your strengths.

Survival tools for a neurodivergent brain in academia
Working in academia is hard, and being neurodivergent makes it harder. Here are a few tools that may help, from a Ph.D. student with ADHD.

Quieting the static: Building inclusive STEM classrooms
Christin Monroe, an assistant professor of chemistry at Landmark College, offers practical tips to help educators make their classrooms more accessible to neurodivergent scientists.

Hidden strengths of an autistic scientist
Navigating the world of scientific research as an autistic scientist comes with unique challenges —microaggressions, communication hurdles and the constant pressure to conform to social norms, postbaccalaureate student Taylor Stolberg writes.
Richard Silverman to speak at ASBMB 2025
Richard Silverman and Melissa Moore are the featured speakers at the ASBMB annual meeting to be held April 12-15 in Chicago.

Women’s History Month: Educating and inspiring generations
Through early classroom experiences, undergraduate education and advanced research training, women leaders are shaping a more inclusive and supportive scientific community.

