MSU researchers leverage cryo-EM for decades-in-the-making breakthrough
When Ben Orlando delivered a 2019 research talk at the Michigan State University Department of Biochemistry and Molecular Biology, it set the stage for a collaborative breakthrough that was decades in the making.

In the lecture hall that day was Professor Emeritus Lee Kroos, who just this September celebrated his retirement from MSU after a pioneering career of over 30 years.
An expert in the molecular and genetic mechanisms of bacteria, Kroos had long worked with Spartan colleagues to capture the elusive structure of a protein known as SpoIVFB (pronounced “spo-four-eff-bee").
Found in the model bacterium Bacillus subtilis, or B. subtilis, SpoIVFB belongs to a family of specialized enzymes known as intramembrane proteases. These enzymes help regulate key cellular functions across all kingdoms of life.
Notably, SpoIVFB plays a critical role in sporulation — a process that allows bacteria to survive harsh conditions such as radiation, heat and even the vacuum of space.
With attempts to capture SpoIVFB’s structure facing unique challenges over the years, Kroos was interested to learn of Orlando’s expertise in the cutting-edge field of cryo-electron microscopy, or cryo-EM.
“I think Ben and I were on the same wavelength,” Kroos said, who reached out about teaming up to solve SpoIVFB’s structure puzzle when Orlando joined the Department of Biochemistry and Molecular Biology, or BMB, in the summer of 2020.
“Ben was very optimistic we could make progress on this thing.”

Now, in a paper appearing in Nature Communications, the Orlando and Kroos research groups report the first high-resolution experimentally determined structures of SpoIVFB.
Specifically, the researchers have revealed SpoIVFB bound to its substrate. Like a key to a lock, substrates are particular molecules that enzymes interact with, facilitating the creation of useful biochemical products.
This study offers insight into a mechanism of cellular regulation found in organisms ranging from bacteria to humans, with exciting implications for microbiology, structural biology, enzymology and human disease.
The breakthrough is also a testament to the ongoing, $15 million expansion of advanced cryo-EM infrastructure at MSU that continues to allow Spartan researchers to push the boundaries of experimental feasibility.
“Over the last several years this technology has been transformative in membrane protein structural biology,” Orlando said, who was recruited by MSU as part of its Global Impact Initiative — a campus-wide undertaking that seeks to tackle grand challenges in energy, health, education and the environment.
“Cryo-EM allows us to peer into a world that we simply can’t see through any other lens.”
The last piece of a biological puzzle
By capturing the structure of SpoIVFB engaging its substrate, the researchers have broadened biochemists’ understanding of a critical biological process.
Proteases typically help break down or “cleave” proteins through proteolysis, a reaction that requires water to sever the peptide bonds between amino acids. However, for a protease like SpoIVFB that exists within a cell’s membrane, this is no small task.
“Going back to the discovery of intramembrane proteases — what some call ‘scissors in the membrane’ — there was no concept this could happen,” Kroos explained.
“The membrane’s lipid bilayer is hydrophobic, and all proteases need water to break peptide bonds. So, the big question became: how does water get in there?”
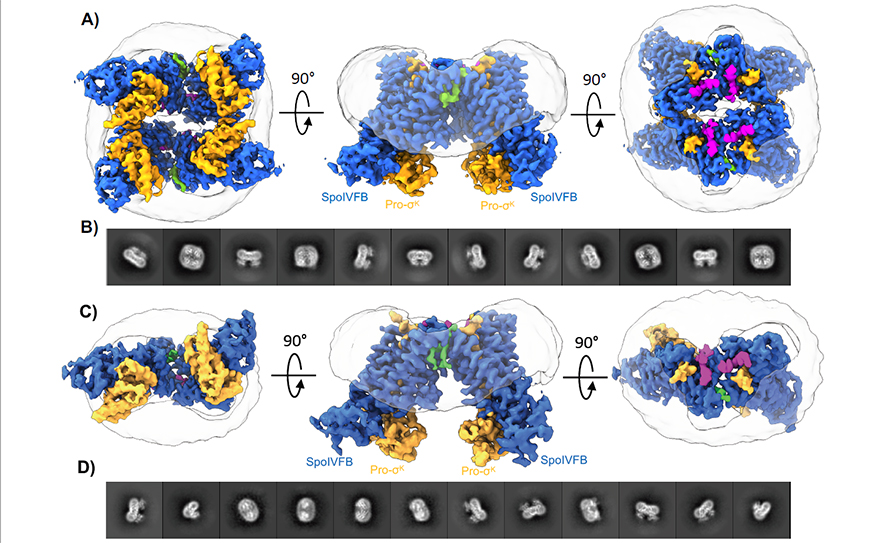
Through cryo-EM imaging and molecular dynamics, Kroos and Orlando confirmed the mechanism SpoIVFB uses to prepare its substrate for cleaving, as well as the route water might take to reach this site.
The cryo-EM structures determined by Orlando and Kroos demonstrate that SpoIVFB engages the substrate through an interaction known as beta-sheet augmentation. This revelation was the final piece of a vast biological puzzle. Four classes of intramembrane proteases are found across the kingdoms of life, from the simplest bacterium to humans. Three of these were known to engage their substrates through beta-sheet augmentation.
With their latest discovery, the MSU team has finally confirmed the fourth.
“These structures link the beta-sheet augmentation mechanism to all four classes of intramembrane proteases, operating as a common mechanism across the kingdoms of life,” Orlando said.
These findings contribute critical knowledge to structural biology, as well as human health.
The failure of intramembrane proteases to regulate cellular functions has been linked to neurodegenerative diseases, cancers, and metabolic disorders.
“There are many indications that these intramembrane proteases also influence the virulence of important pathogenic bacteria,” Kroos added. “If we know their structure precisely, we could design antimicrobials for important diseases.”
The team’s findings are likewise a fitting capstone to a 36-year career at BMB and the microbiology, genetics and immunology department for Kroos, who was recently spotted enjoying his retirement fly-fishing on the Pere Marquette River.
“Over the past few years, collaborating with Ben has been awesome,” Kroos said. “We’ve had wonderful, in-depth conversations on this work — it’s been a real high note.”
From atoms to organisms
In the years-long quest to characterize SpoIVFB, Kroos and fellow BMB colleagues had first applied a method known as X-ray crystallography. Here, X-rays are directed at a crystallized sample to create a diffraction pattern, ultimately revealing t3D molecular structure.

The quality of SpoIVFB’s crystals and the quantity of sample required, however, were two consistent hurdles.
“SpoIVFB was very resistant to our crystallization efforts, and it was difficult to make significant quantities that were active and could be used,” Kroos explained.
“Coaxing proteins to coalesce into crystals is challenging enough,” Orlando added. “But when you start talking about membrane proteins like SpoIVFB, you’ve got even more complexity.”
During their attempts at crystallization, the Kroos Group — in collaboration with BMB colleagues such as Michael Garavito, Jian Hu and Michael Feig — continued to make foundational discoveries that ultimately paved a path toward solving SpoIVFB’s structure.
Through their latest collaboration, cryo-EM allowed Orlando and Kroos to bypass some of X-ray crystallography’s biggest bottlenecks and visualize SpoIVFB like never before. In single particle electron microscopy, a beam of electrons is used to take 2-D images of individual protein molecules. These 2-dimensional images are then utilized to generate a 3D map of the target protein.
While offering atomic-scale resolution images of many materials, these same electrons will damage or destroy biological samples. This hurdle led to the cryogenic applications of cryo-EM — an innovation recognized with the 2017 Nobel Prize in Chemistry.
To counter biological damage, cryo-EM samples are prepared in a thin layer of aqueous buffer that is rapidly plunged into liquid ethane to generate a “glass-like” ice. Rather than needing to be arranged into a crystal lattice for analysis, individual particles are locked into various orientations at the time of freezing.
By taking thousands or sometimes millions of images of a sample at different angles, researchers are able to create a highly detailed 3D map of the sample that is being imaged.
“This technology helped get us over that mountain, and as it improves, we’ll peer deeper and deeper into new areas of biology,” Orlando said, who looks forward to what the expansion of MSU’s cryo-EM facility will mean for researchers working across an array of disciplines, from materials science to microbiology and biochemistry.
“I think the real power of a place like this comes from the people across our campus who can utilize the exact same technology and its breadth of scale. It’s the only methodology that I am aware of where we can view it all, from atoms to organisms,” Orlando added.
This article is republished from MSU Today. Read the original here.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

