JLR: Secrets of fat and the lymph node
When you eat a high-fat meal, your gut exports the fat into chylomicrons, which join the flow of lymph in the surrounding vessels. This combined fluid, which resembles cream, passes rapidly through the lymph nodes and into the blood stream, where the fat is absorbed by cells that need energy, like heart muscle cells, or that can store fat for the long term, like adipose tissue.
Sander Kersten, a professor and department chair at Wageningen University in the Netherlands, studies that rapid uptake system, which depends on a protein called lipoprotein lipase, or LPL for short. LPL breaks down triglyceride fat molecules, allowing them to be absorbed. Kersten’s latest study in the Journal of Lipid Research shows that LPL regulation varies among tissues.
Too much fat in the blood can cause problems such as heart disease. Therefore, right after a meal, it is beneficial for adipose tissue to absorb fat rapidly for storage. That means bumping up LPL levels. But during a long fast, limited LPL activity in adipose tissue helps ensure that fat stays available to cells that need it for energy. It is important that our bodies can tune LPL activity in different systems in response to how much food we eat.
Some 20 years ago, as a postdoc, Kersten isolated a protein, ANGPTL4, that acts as a control dial for LPL. Later, his lab demonstrated that the protein targets LPL for degradation in adipose cells. Researchers since have found that ANGPTL4 fluctuates in response to fasting, cold exposure and exercise, helping to control the body’s lipid use.
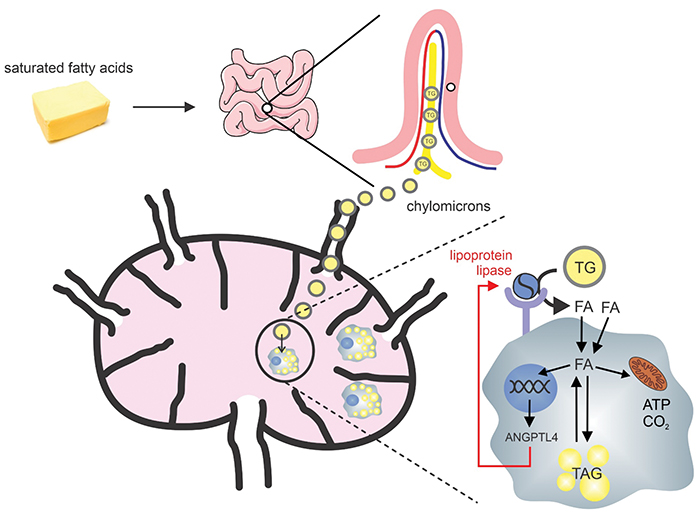 A cartoon Kersten uses for teaching shows how saturated fat from the diet enters the lymph node as chylomicrons and how lymph node resident macrophages respond to the fat.Courtesy of Sander KerstenDrug developers hoped that reducing ANGPTL4 would be a good way to reduce the risk of heart disease, but this research hit a snag. Mice that were bred to have no ANGPTL4 appeared healthy at first, but that health was fragile.
A cartoon Kersten uses for teaching shows how saturated fat from the diet enters the lymph node as chylomicrons and how lymph node resident macrophages respond to the fat.Courtesy of Sander KerstenDrug developers hoped that reducing ANGPTL4 would be a good way to reduce the risk of heart disease, but this research hit a snag. Mice that were bred to have no ANGPTL4 appeared healthy at first, but that health was fragile.
“If you place these animals on a diet that’s rich in fat, they develop complications which were unanticipated,” Kersten said. Lymph carrying chylomicrons escapes into their abdomens, eventually killing the mice. Whether this would happen in humans if you blocked their ANGPTL4 isn’t known — it’s an experiment no one is willing to risk.
The Kersten lab’s latest paper examines why loss of ANGPTL4 has this effect. The work focuses on macrophages, the cells that populate the lymph node. Like fat cells, macrophages express ANGPTL4, and like fat cells, they turn it up in response to high fat in the bloodstream. But ANGPTL4 in macrophages appears to work differently than in fat cells. Although ANGPTL4 reduces LPL activity and fat uptake in macrophages, it doesn’t seem to alter LPL level — suggesting that it does not act by targeting LPL for degradation but by another mechanism. Exactly how ANGPTL4 affects macrophages, Kersten said, remains to be determined.
“After 20 years of studying ANGPTL4, there are some things that are very, very clear about this protein,” Kersten said. “And I’m happy to have contributed to that.”
Other questions remain. “We’re not still 100% sure about what is going on in the lymph nodes.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

Redefining excellence to drive equity and innovation
Donita Brady will receive the ASBMB Ruth Kirschstein Award for Maximizing Access in Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Mining microbes for rare earth solutions
Joseph Cotruvo, Jr., will receive the ASBMB Mildred Cohn Young Investigator Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

