Crystal building blocks of triglycerides
Francis Crick once said, “If you want to understand function, study structure.” Do you agree? I certainly do, and I would argue that most lipid biologists do too. Just consider the effort to define chemical structures for the many thousands of lipids that exist and the hypotheses about individual lipid function that this structural information has generated.
What has lagged behind is the characterization of the structures of the proteins that modify, transport or interact with these lipids, but times are changing. For example, when I started my postdoc in Yusuf Hannun’s lab, only a handful of sphingolipid-metabolizing enzymes had been structurally characterized, and these were mainly from bacteria. While many questions remain open (hey, ceramide synthase — we can’t wait to see what you look like!), work from several labs has defined the structures and mechanisms for many human enzymes in sphingolipid metabolism.
A similar revolution appears to be happening with triglycerides. As most of you know, triglycerides serve as a reservoir for energy storage, but when they accumulate excessively, they can cause health problems, including obesity, diabetes and heart disease. Three new structures in particular have caught my attention.
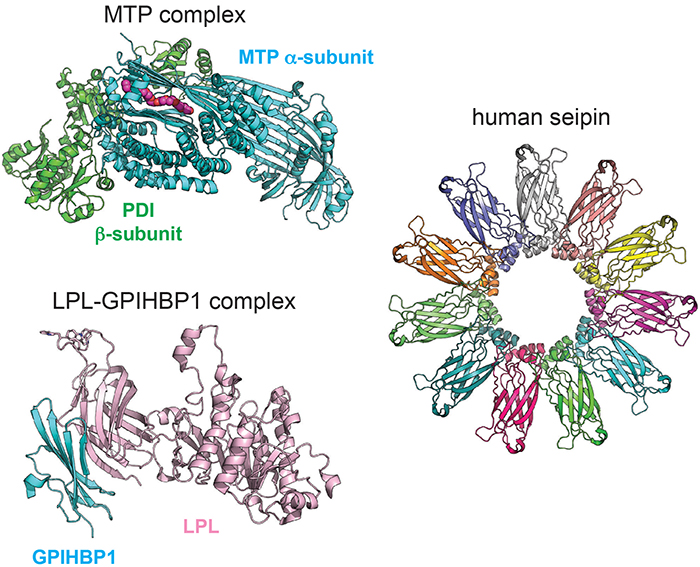 Beautiful structures of proteins involved in triglyceride metabolism and storage.Michael AirolaThe most recent is a crystal structure of microsomal triglyceride transfer protein complex, which transfers neutral lipids into apolipoprotein B-containing lipoproteins. The arduous crystallography required to conduct this work is impressive. The researchers revealed an unexpected lipid-binding cavity and provided insight into disease mutations as well as pharmacological inhibition of this therapeutic target.
Beautiful structures of proteins involved in triglyceride metabolism and storage.Michael AirolaThe most recent is a crystal structure of microsomal triglyceride transfer protein complex, which transfers neutral lipids into apolipoprotein B-containing lipoproteins. The arduous crystallography required to conduct this work is impressive. The researchers revealed an unexpected lipid-binding cavity and provided insight into disease mutations as well as pharmacological inhibition of this therapeutic target.
The second is the crystal structure of lipoprotein lipase, or LPL, the major lipase that clears triglycerides in the blood. Gabriel Birrane and colleagues and Risha Arora and colleagues separately determined the LPL structures, overcoming the relative instability of LPL by complexing it with its binding partner glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. These structures, along with other biochemical data, suggest LPL is active as a monomer, challenging the long-standing paradigm that LPL was only active as a dimer.
The last notable structure is that of seipin, a homo-oligomeric integral membrane protein that is a key player in the formation of cytoplasmic lipid droplets. Two groups (Renhong Yan and colleagues and Xuewu Sui and colleagues), using cryo-electron microscopy, found that 11 or 12 seipin molecules (dependent on the species) come together to form a ring that spans the endoplasmic reticulum membrane, can bind phosphatidic acid and may stabilize the formation of nascent lipid droplets.
What’s next? Who knows, but I’m darn sure we’re all gonna love it.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

Redefining excellence to drive equity and innovation
Donita Brady will receive the ASBMB Ruth Kirschstein Award for Maximizing Access in Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

