An essential debate
Is a particular dietary recommendation harming people in the U.S.? For almost 20 years, scientists have been arguing over whether Americans and others on a typical Western diet are eating too much of omega-6s, a class of essential fatty acids. Some experts, notably ones affiliated with the American Heart Association, credit our current intake of omega-6s with lowering the incidence of cardiovascular disease. Others, which include biochemists, say the relatively high intake of omega-6 is a reason for a slew of chronic illnesses in the Western world, including asthma, various cancers, neurological disorders and cardiovascular disease itself.
.jpg?width=800&height=400)
At the center of this dispute is how omega-6s and their cousins, omega-3s, are biochemically processed in the body and the physiological outcomes of the metabolism (see box on the EFA naming convention). Both camps agree that a healthy diet requires both omega-3s and omega-6s. Omega-3s are sorely lacking in Western diets, so people need to increase their consumption of them. But the split comes over omega-6s: Are we or are we not eating too much of them?
To make their case, both camps point to the same body of biochemical and clinical trial data but say the other camp is misinterpreting the data. “It’s a real can of worms,” says Norman Salem Jr., a biochemist at DSM Nutritional Products, a company that makes essential fatty acids and other food supplements.
Opening the can of worms
In 1985, Artemis Simopoulos, then the chair of the nutrition coordinating committee of the National Institutes of Health and now the president of the Center for Genetics, Nutrition and Health, described how, from the 1950s onward, Western diets were becoming dominated by omega-6s at the expense of omega-3s. Fish intake, a critical source of omega-3s, had dropped considerably, even among American Catholics after Pope Paul VI decreed in 1966 that Fridays no longer had to be meatless (1). “By 1985, fish consumption was minimal. Something like 25 percent of the U.S. population did not eat any fish at all,” says Simopoulos. “In the meantime, agriculture and agribusiness had changed animal feeds. The omega-3 fatty acids that are normally found in grass-eating animals had disappeared because the animals were fed corn.” Corn and other grains are generally high in omega-6s.
The oils people consumed also changed. People began to eat more corn, soybean and safflower oils, which are high in omega-6s and plentiful in industrially processed foods. Another source of the dietary change came from the work of Ancel Keys. In 1965, Keys and his colleagues at the University of Minnesota published an equation that quantified the relationship between saturated fats, polyunsaturated fats and serum cholesterol levels (2). The equation helped to sway public health dietary emphasis toward the polyunsaturated fats, which include EFAs, to lower blood cholesterol levels. But the Keys equation treated all unsaturated fats the same. It didn’t distinguish between omega-3s and omega-6s. But biochemical studies, such as those by Nobel laureates Bengt Samuelsson and Sune Bergström at the Karolinska Institute in Sweden and John Vane at the Wellcome Foundation in the U.K., were demonstrating that the two classes of EFAs formed different kinds of bioactive eicosanoids.
In 2009, the dispute over omega-6 consumption came to a head when the American Heart Association recommended that omega-6s comprise at least 5 percent to 10 percent of the energy intake in a diet (3). If omega-6 intakes were less than that, said the AHA, the risk of cardiovascular disease likely would increase. According to data from the National Health and Nutrition Examination Survey overseen by the Centers for Disease Control and Prevention, the average American currently gets 6.5 percent of his or her energy intake from linoleic acid, the major omega-6 in the diet.
William Harris, an expert in cardiovascular disease at the University of South Dakota and president of the company OmegaQuant Analytics, says that much of the evidence, both from dietary studies and measurements of blood levels, has shown that higher blood levels of omega-6 are associated with reduced risks for cardiovascular events. Harris, who spearheaded the heart association’s scientific advisory group, adds that this is why “the American Heart Association encourages people to eat omega-6 fatty acids and specifically not to buy into this idea that we’re eating too much.” Walter Willett, an expert in nutrition and epidemiology at Harvard University, goes one step further and calls the assertion that high omega-6 intake is harmful “an urban myth.”
But lipid biochemists are alarmed. For example, Floyd Chilton is a biochemist at Wake Forest Baptist Medical Center and a consultant to the company GeneSmart. He says that the AHA recommendation was made without considering the biochemistry of EFAs and solely focused on the outcomes of clinical trials that were not well designed. “I think it led to some very troubling decisions,” he says, a sentiment echoed by others, such as Simopoulos, Salem and William Lands, a biochemist at the National Institute on Alcohol Abuse and Alcoholism.
The biochemical debate
Essential fatty acids were discovered on the heels of vitamins in the early 1900s. In 1929 and 1930, George and Mildred Burr demonstrated that essential fatty acids were critical for the well-being of laboratory rats and discovered the first essential fatty acid, the omega-6 linoleic acid (4). In 1933, one of George Burr’s graduate students, Arild Hansen, showed that humans, like the laboratory rats, could suffer from deficiencies in essential fatty acids. Soon both omega-3s and omega-6s were accepted as nutrients like vitamins that had to be consumed through food for optimal health.
EFAs have several functions, which include producing bioactive molecules, making up our tissue composition and contributing to the skin’s barrier function. EFAs go through one well-documented biochemical pathway to make a class of bioactive molecules called eicosanoids. This pathway is also a source of contention between the two camps.
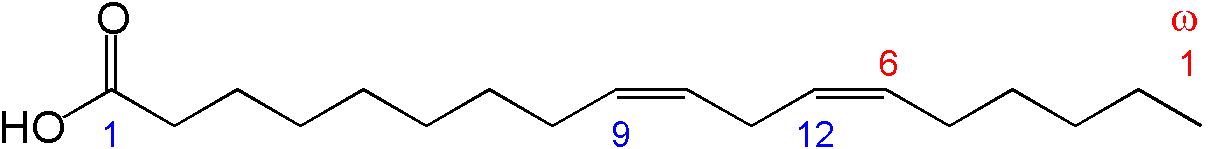 Chemical structure of linoleic acid Wikipedia
Chemical structure of linoleic acid Wikipedia
Linoleic acid, the main omega-6, enters the pathway through the Δ6 desaturase. The resulting molecule then gets elongated by an enzyme whose identity has not yet been established. Next, the molecule goes through the Δ5 desaturase. The result is arachidonic acid, a 20-carbon chain that gets converted by cyclooxygenases into eicosanoids, such as leukotrienes, prostaglandins, and thromboxanes, which regulate inflammation and thrombosis. (Aspirin and its fellow nonsteroidal anti-inflammatory drugs inhibit cyclooxygenases.)
α-linolenic acid, an omega-3 fatty acid, also enters the biochemical pathway through the Δ6 desaturase and is transferred to the Δ5 desaturase. The product from the Δ5 desaturase, eicosapentaenoic acid, goes on to form docosahexaenoic acid through additional steps involving elongases. EPA and DHA, like arachidonic acid, help to build eicosanoids. But these eicosanoids are not as potent as the ones made from arachidonic acid.
Here is the contention between the two camps. The camp that believes we eat too much omega-6 says that linoleic acid swamps out the biochemical pathway, not giving α-linolenic acid much of a chance to get to the Δ6 desaturase. All you get with the high linoleic acid amount, argues this camp, is an accumulation of the powerful arachidonic acid, which shifts the body into a state of constant inflammation. But the camp that argues that our omega-6 intake is fine gets frustrated by this argument. Penny Kris-Etherton, a nutrition expert at Pennsylvania State University who was in Harris’ advisory group, says the conversion issue goes away if people just eat adequate amounts of EPA and DHA. If EPA and DHA are sufficient in the diet, the need to convert α-linolenic acid into those molecules gets bypassed. “Just eat a lot of fish and we don’t have to worry about the conversion issue,” she says. Fortified foods that contain EPA and DHA, like milk and eggs, can also be sources of omega-3s.
Even then, says Salem, the products of arachidonic acid compete against those from EPA and DHA for incorporation into complex lipids and into cells and organs. Indeed, arachidonic acid is a better substrate for cyclooxygenases than EPA. William Smith, a biochemist at the University of Michigan at Ann Arbor, says that cyclooxygenase 1 uses EPA at 5 percent to 10 percent of the efficiency of arachidonic acid; cyclooxygenase 2 uses EPA at a somewhat greater efficiency but no more than 20 percent to 30 percent. “If you have a fairly high level of arachidonic acid over EPA, you’re opening the floodgates for all the things that prostaglandins do,” which includes stimulating inflammation, says Smith.
Even if products from arachidonic acid dominate, Harris argues, products of arachidonic acid can’t all be painted with the same brush. He says some of the molecules produced by omega-6s are proinflammatory but some are anti-inflammatory. “One can’t certainly say the omega-6s are pro-inflammatory,” states Harris. “That’s far too simple, because there are several examples of omega-6 metabolites that are either anti-inflammatory or antiplatelet aggregation.”
Lands says Harris’ point about omega-6s producing both pro- and anti-inflammatory molecules is correct. But as a whole, he counters, the proinflammatory molecules from omega-6s dominate their anti-inflammatory counterparts. These proinflammatory molecules, Lands, Chilton, and others in the camp advocating lower omega-6 levels assert, accumulate in the body and result in several diseases for which inflammation of some sort is the root cause.
Genetics also now has entered the fray. In recent years, Chilton’s laboratory has been studying the FADS cluster, which codes for the Δ5 and Δ6 desaturases (5, 6). His team has shown that people of African descent have much higher frequencies of genetic variants that efficiently convert linoleic acid to arachidonic acid than people of European descent. As a result, African-Americans have much higher circulating blood levels of arachidonic acid, says Chilton.
This difference in conversion rate is important for an assertion often made by those advocating for the current omega-6 levels. They say that the biochemical pathway self-regulates, turning itself off after a certain amount of arachidonic acid is made. “If we eat more omega-6s in the diet, it doesn’t increase arachidonic acid levels, because there is so much regulation,” says Willett.
But Chilton says his research suggests an individual’s background does matter. “Many people have argued that the system saturates itself at relatively low concentrations of dietary linoleic acid, limiting the amounts of arachidonic acid that can be made,” he says. But, he adds, the studies were almost exclusively carried out in European and European-ancestry populations. Given that individuals of African descent have higher frequencies of the variants that convert linoleic acid to arachidonic acid at higher rates than European descendants, Chilton says it is likely that the saturation of the linoleic–acid-to-arachidonic-acid pathway occurs at higher levels of dietary linoleic acid in people of African descent. “With recommendations like that from the American Heart Association, I am particularly concerned we may be driving health disparities,” says Chilton.
Lands and other biochemists decry what they see as a complete dismissal of the biochemistry of essential fatty acids in making dietary recommendations. But to that critique, Harris says public health policy should be dictated by clinical trial data. “You don’t do it by looking at biochemical pathways,” he says. “You look at randomized controlled trials and population cohort studies where disease endpoints are being measured. That’s what you care about.”
Clinical conundrum
In coming up with its 2009 recommendation, the AHA looked at the literature describing various clinical trials that included omega-6s. The AHA used evidence from observational trials that lasted up to 20 years and randomized controlled trials in which participants were given special diets. Subsequently, biochemist Joseph Hibbeln and clinician Christopher Ramsden at the National Institute on Alcohol Abuse and Alcoholism analyzed the oils used in the randomized controlled clinical trials (7). They asked if the evidence for the AHA recommendation was specific to linoleic acid or if an increase in omega-3s could be responsible for the benefits.
Ramsden and Hibbeln describe the problems they see: The trials involving only omega-6 didn’t give any indication of benefit and even suggested a hint toward increased risk; only the trials that increased omega-3s along with omega-6s presented a benefit. The AHA, they say, did not separate the trials involving only omega-6s from those that also increased omega-3s.
“Here’s the analogy,” says Hibbeln. “Say I give marshmallows infused with penicillin to people who have an infection and they get better. By gram weight, the intervention was mostly marshmallows. But there was penicillin. Do I then overgeneralize, as the American Heart Association has done, and recommend that people eat marshmallows to cure their infections? I don’t think so.”
Harris doesn’t buy that argument. Biochemistry dictates that an excess of linoleic acid should suppress the conversion of α-linolenic acid to EPA and DHA. It must be linoleic acid that is the main factor in preventing cardiovascular disease in these clinical trials, Harris says, adding that the molecule is known to have beneficial effects, such as lowering low-density lipoprotein cholesterol. The onus is still on Hibbeln’s group “to explain how a small amount of α-linolenic acid contained in a virtual ocean of linoleic acid” can have cardioprotective effects, says Harris.
But Ramsden counters that the one trial that increased the daily intakes of EPA and DHA to 5 grams, a large amount equivalent to 16 fish-oil pills a day, showed major benefit. If that trial, called the Oslo Diet Heart Study, was excluded from the meta-analysis, none of the other clinical trials showed a benefit from the omega-6s.
There aren’t any data showing what happens when linoleic acid in people’s diets is dropped to less than 5 percent, an amount at which it’s thought that linoleic acid doesn’t crowd out α-linolenic acid. What’s needed is a clinical trial that carefully tracks a large range of linoleic acid amounts in the diet and catalogues health outcomes.
“That trial will never be done. It would take 10 years or longer and cost perhaps $100 million,” says Salem. “No one is ever going to pay for that trial.” Harris concurs, saying the randomized, controlled trial would be extremely hard to pull off, because the entire food supply would have to be manipulated to test different amounts of linoleic acid.
“We’re the experiment”
No one is disputing that we’re eating more omega-6 than our predecessors did. Over the past 100 years, consumption of linoleic acid has increased dramatically in the U.S., mainly through the use of soybean oil. Soybean oil intake has gone up from being 1 percent of calories in the American diet to as much as 10 percent, according to Hibbeln. Lands, Salem and others contend that the rise, driven by the processed food and agriculture industries, has happened without anyone knowing its effects. “If I were now to try to get permission to change 10 percent of the calories in the U.S. diet, I would need a very large body of data unequivocally proving that it was safe,” says Hibbeln. “No such body of data exists for soybean oil. But it’s in our diet. We’re the experiment. It’s been a very large, uncontrolled intervention.”
Experts like Harris and Willett say this increase has been to our benefit. “We have seen a massive decline in cardiovascular disease mortality and huge increase in life expectancy,” says Willett. “Not all the benefit is due to the increase in linoleic acid, but almost certainly much of it is. It was not an absolute disaster.” But the lipid biochemists counter that it’s not just cardiovascular disease at stake. They say diabetes, obesity and even psychiatric disorders are some outcomes of a diet heavy on omega-6s.
Both sides agree that there is much more research to be done on the various pathways in which EFAs participate. Ramsden says nonspecialists may have the impression, based on the well-known body of work on prostaglandins and a few other categories of compounds derived from EFAs, that the biochemistry of omega-6s and omega-3s is firmly established. But that is not the case, because “it’s really more complex than anyone had ever thought.” Ramsden continues: “I look at this field as having a long way to go.”
For this reason, the current experts in the field urge more scientists and clinicians to join in the efforts to understand the impact of EFAs on health outcomes and perhaps finally lay the omega-6 dispute to rest. As Harris says, “The more people are tuned into the question, the more research will be done on the topic and the less uncertainty we will have around the issue.”
References
- Bell, F. W. Amer. Econ. Rev58, 1346–1350 (1968).
- Keys A., et al. Metabolism14, 776–787 (1965).
- Harris, W. S. et al. Circulation119, 902–907 (2009).
- Mukhopadhyay, R. J. Biol. Chem. doi: 10.1074/jbc.O112.000005 (2012).
- Sergeant, S. et al. Br. J. Nutr. 107, 547–555 (2012).
- Mathias, R. A. et al. BMC Genet. doi: 10.1186/1471-2156-12-50 (2011).
- Ramsden, C. E. et al. Br. J. Nutr. 104, 1586–1600 (2010).
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

Exploring lipid metabolism: A journey through time and innovation
Recent lipid metabolism research has unveiled critical insights into lipid–protein interactions, offering potential therapeutic targets for metabolic and neurodegenerative diseases. Check out the latest in lipid science at the ASBMB annual meeting.

Melissa Moore to speak at ASBMB 2025
Richard Silverman and Melissa Moore are the featured speakers at the ASBMB annual meeting to be held April 12-15 in Chicago.

