Top-notch papers by postdocs
National Postdoc Appreciation Week is Sept. 19–23 — a time to celebrate postdocs and their immeasurable contributions to the scientific community. The American Society for Biochemistry and Molecular Biology participates in this observance, which is led by the National Postdoctoral Association, each year.
In recognition of postdocs’ hard work and dedication to the scientific enterprise, here we highlight some of the postdoc first authors of the most-read papers in the ASBMB journals — the Journal of Biological Chemistry, Journal of Lipid Research, and Molecular & Cellular Proteomics.
Journal of Biological Chemistry
Goldilocks calcium concentrations and the regulation of oxidative phosphorylation: too much, too little, or just right


Eloisa Vilas Bôas is a postdoctoral fellow at the University of São Paulo in Brazil and conducts research with Alicia Kowaltowski, a professor of biochemistry.
In her JBC publication, Vilas Bôas investigated the role of mitochondrial calcium concentrations on oxidative phosphorylation and ATP production. Calcium is the most abundant mineral in the human body, said Vilas Bôas. She found that cells maintain cellular and mitochondrial calcium concentrations within strictly controlled ranges, maximizing electron transport capacity by promoting what they referred to as the Goldilocks” calcium sweet spot for oxidative phosphorylation activity. She said that if there is a lack or excess of calcium, oxidative phosphorylation is limited, while the “just right” concentration promotes significant ATP production. Her future work will focus on understanding the effects of hormones on mitochondrial respiration and calcium signaling.
Vilas Bôas overcame challenges associated with the COVID-19 pandemic to publish this study. In addition, she said that “as Brazilian scientists, we face little financial aid from government or private entities, so we always have to optimize resources.”
Mitochondrial respiration reduces exposure of the nucleus to oxygen
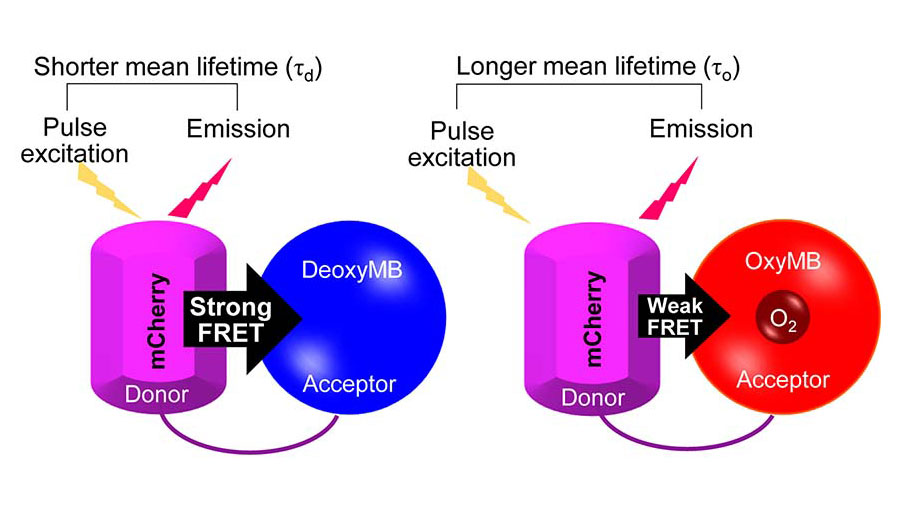

Mateus Prates Mori is a postdoctoral visiting fellow at the National Institute of Environmental Health Sciences and conducts research with group leader Janine Santos. At the time of this publication, Prates Mori was a postdoctoral fellow in the lab of Paul Hwang, a senior investigator at NHLBI.
Prates Mori’s paper examined the oxygen concentrations in various compartments of the cell such as the cytosol, mitochondria and nucleus. He found that the mitochondria and the nucleus contain the smallest concentration of oxygen, and mitochondrial respiration creates an intracellular compartmental oxygen gradient. In the future, Prates Mori said he wants to uncover novel oxygen-sensitive targets in the nucleus in the context of mitochondrial dysfunction.
While he was conducting this research, Prates Mori and his spouse welcomed their first child. He said he had to learn to balance his work and personal life, which was especially difficult because his relatives live outside of U.S. “These are the events that happened behind the scenes that are usually never mentioned or acknowledged but are far more challenging than those of our daily routine of experiments,” Prates Mori said.
Liquid–liquid phase separation of amyloid-β oligomers modulates amyloid fibrils formation
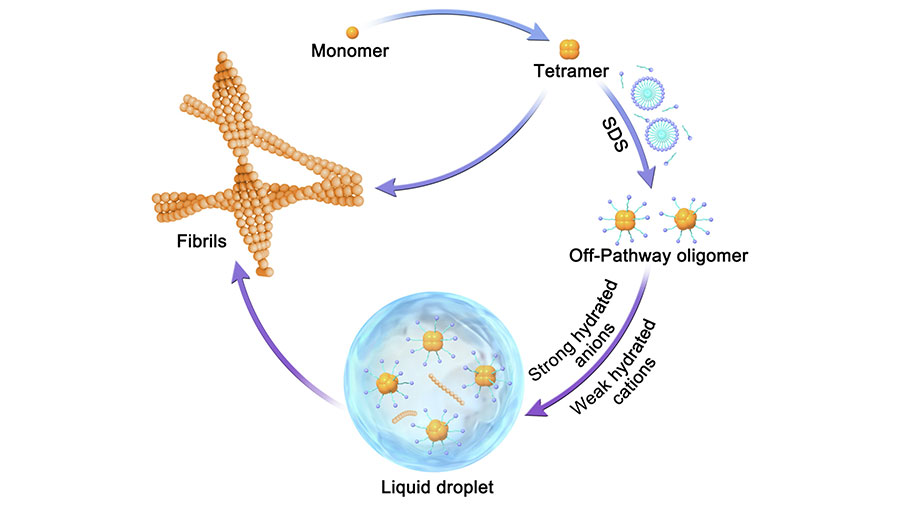

Xinrui Gui is a postdoctoral fellow at St. Jude Children's Research Hospital in the lab of Tanja Mittag, a member of the structural biology departmen. At the time of this publication, Gui was a staff member at Henan University in Kaifeng, China.
Gui’s publication examined how toxic amyloid-β oligomers, a pathogenic species in Alzheimer’s disease, drive protein phase separation and fibrillization. She found that amyloid-β oligomers undergo phase separation in vitro and that amyloid-β oligomer liquid–liquid phase separation modulates a liquid-to-solid phase transition and redirects the aggregation pathway to form pathogenic amyloid fibrils. This research revealed the regulatory role of liquid–liquid phase separation that underlies amyloid protein aggregation and may inform future Alzheimer’s disease therapeutics. Her future research will focus on the structural changes of amyloid-β oligomers during phase separation.
During her project, Gui said, she screened many conditions to stabilize amyloid-β oligomer formation and phase separation in vitro, which was critical for the success of the project.
Journal of Lipid Research
Combining ASBT inhibitor and FGF15 treatments enhances therapeutic efficacy against cholangiopathy in female but not male Cyp2c70 KO mice
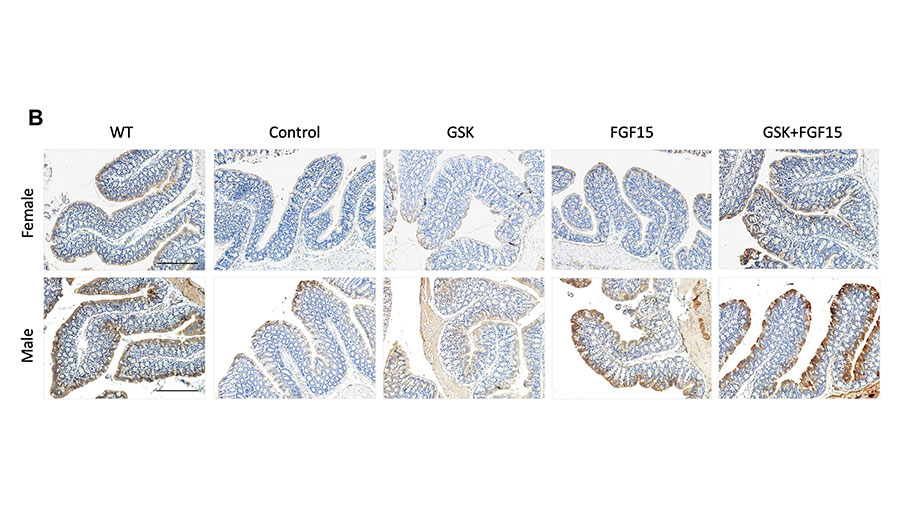

Mohammad Nazmul Hasan is a postdoctoral fellow at the University of Oklahoma Health Sciences Center in the lab of Tiangang Li, a professor of physiology.
Hasan used a mouse model of cholestasis, a chronic liver disease, to investigate potential therapeutics in vivo. His research showed that combining an apical sodium-dependent bile acid transporter inhibitor and fibroblast growth factor-15 overexpression alleviated cholestasis in female, but not male, mice by reducing bile acid pool size and hydrophobicity compared to monotherapy. Hasan’s research could provide a conceptual framework for developing new therapeutic strategies for treating human cholestasis. His future work will dissect the mechanisms underlying the sex-dependent treatment outcomes.
Hasan’s postdoctoral fellowship allowed him to specialize in a new field: liver metabolism. “My postdoctoral training helped me think independently, improve my skills and report new findings to the scientific society,” he said. “Guidance from my mentor and collaborations with my colleagues are important for me to be successful in my research.”
Acetyl-CoA carboxylase inhibitor increases LDL-apoB production rate in NASH with cirrhosis: prevention by fenofibrate


Mohamad Dandan is a postdoctoral fellow in the lab of James Fraser, the vice dean of research and a professor of bioengineering and therapeutic sciences at the University of California, San Francisco. At the time of this publication, he was working with Marc Hellerstein, a professor of nutritional sciences and toxicology at UC Berkley.
For this study, Dandan collaborated with Gilead Sciences on a phase II clinical trial examining the effect of acetyl-CoA carboxylase inhibitors on lipoprotein metabolism in patients with nonalcoholic steatohepatitis, or NASH. The team showed that NASH patient treatment with acetyl-CoA carboxylase inhibitors increased lipoprotein particle apoB100, the main structural protein of very low-density lipoprotein particles, using metabolic labeling with heavy water and tandem mass spectrometric analysis. However, this undesirable outcome can be overcome using combination therapy with fenofibrate, a Food and Drug Administration approved drug that lowers LDL levels. Future directions of the project will examine the flux of proteins during disease progression for potential use as biomarkers.
Dandan said he was very grateful to his mentor and Gilead for the experience of working on a clinical trial. “My research is only one example of how we can help clinicians, chemists, big pharma and physiologists make informed decisions on how to navigate the complexity of biology,” he said. “I envision my research will extend into not only physiology but to basic bench science in cellular and molecular physiology.”
Molecular & Cellular Proteomics
Proteomic analysis of Huntington’s disease medium spiny neurons identifies alterations in lipid droplets


Kizito-Tshitoko Tshilenge is a postdoctoral fellow in the lab of Lisa Ellerby, a professor at the Buck Institute for Research on Aging in California.
The goal of his research project was to examine the proteome of striatal neurons, the cells that are lost in the brains of Huntington’s disease patients. Tshilenge generated striatal neurons from human induced pluripotent stem cells that harbored the mutation that causes Huntington’s. With their colleagues, they used mass spectrometry technology which produced a very comprehensive proteome dataset. Using a combination of bioinformatic approaches, immunocytochemistry and confocal imaging, the team found that lipid droplets accumulate in striatal neurons with the Huntington’s mutation. The next steps of Tshilenge’s research will explore how dysregulated lipid metabolism in striatal neurons contributes to Huntington’s pathology.
Tshilenge said that having a human proteomic dataset that recapitulates Huntington’s disease will be valuable to the research community and advance the field. “The proteome of the striatal neurons of human Huntington’s disease is likely to provide the path to develop potential therapeutic targets or biomarkers for Huntington’s patients.”
Sensitive, high-throughput HLA-I and HLA-II immunopeptidomics using parallel accumulation–serial fragmentation mass spectrometry
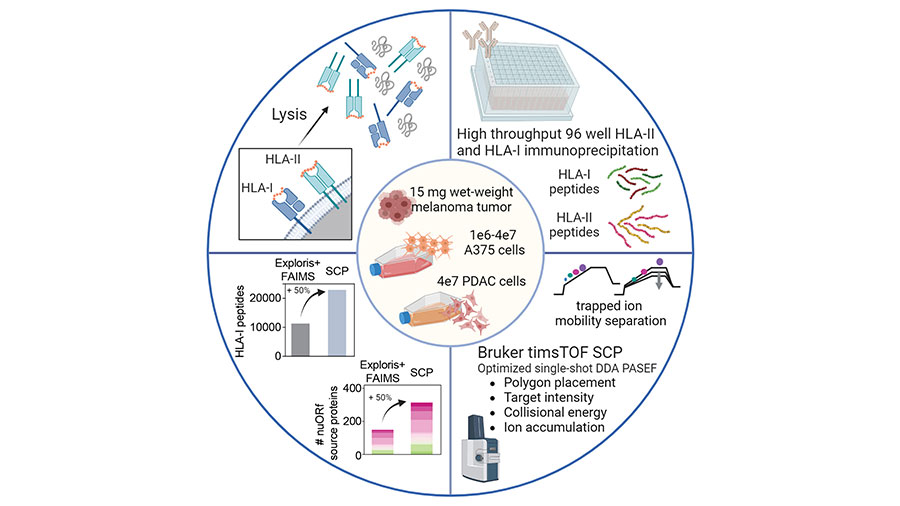


Kshiti Meera Phulphagar and Claudia Ctortecka are postdoctoral fellows in the lab of Steven Carr, the senior director of proteomics and an institute scientist at the Broad Institute and deputy editor of MCP.
Phulphagar and Ctortecka wanted to improve mass spectrometry–based immunopeptidomics to identify clinically relevant, novel tumor antigens from a very small amount of patient sample. The team developed and applied a high-throughput, sensitive, and single-shot mass spectrometry-based immunopeptidomics workflow, which requires less than one-tenth the amount of sample and yields two-fold improved coverage of tumor immunopeptidomes compared to current methods. The researchers are currently working on further optimizing the method to examine atypical peptides.
Phulphagar and Ctortecka worked together to bring this project to fruition as co-first authors. They both said this pipeline could increase access to patient genomic profiling technology. “Our hope is that new, improved methods such as ours will aid the discovery of new tumor specific peptide antigens presented in various different cancers, eventually leading to personalized and more effective cancer therapies with reduced side effects,” Phulphagar said.
Ctortecka added: “I strongly believe that the more meaningful and complementary data can be generated from a patient or a patient cohort, the better-informed treatment decisions can be.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Building better tools to decipher the lipidome
Chemical engineer–turned–biophysicist Matthew Mitsche uses curiosity, coding and creativity to tackle lipid biology, uncovering PNPLA3’s role in fatty liver disease and advancing mass spectrometry tools for studying complex lipid systems.

Redefining lipid biology from droplets to ferroptosis
James Olzmann will receive the ASBMB Avanti Award in Lipids at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Women’s health cannot leave rare diseases behind
A physician living with lymphangioleiomyomatosis and a basic scientist explain why patient-driven, trial-ready research is essential to turning momentum into meaningful progress.

Life in four dimensions: When biology outpaces the brain
Nobel laureate Eric Betzig will discuss his research on information transfer in biology from proteins to organisms at the 2026 ASBMB Annual Meeting.

Fasting, fat and the molecular switches that keep us alive
Nutritional biochemist and JLR AE Sander Kersten has spent decades uncovering how the body adapts to fasting. His discoveries on lipid metabolism and gene regulation reveal how our ancient survival mechanisms may hold keys to modern metabolic health.

