Play by the rules — and spot the error
Over the course of this year, I’ve offered advice on figure presentation and assembly. For this month’s installment of Due Diligence, I thought it would be helpful to discuss the American Society for Biochemistry and Molecular Biology’s expectations for creating technically and ethically sound figures as well as caution against some common image adjustment errors. I’m also letting you, the reader, play reviewer by showing you several figures (below) to see if you can spot the presentation error.
To ensure the integrity of the papers they publish, many journals provide guidance for acceptable practices when it comes to image manipulation. These standards were introduced in response to an increase in inappropriate adjustments of images in figures. Most journals have adopted the Journal of Cell Biology’s stance on image manipulation because it’s thorough and rigorous:
“No specific feature within an image may be enhanced, obscured, moved, removed, or introduced. The groupings of images from different parts of the same gel fields or exposures must be made explicit by the arrangement of the figure (e.g., using dividing lines) and in the text of the figure legend. Adjustments of brightness, contrast, or color balance are acceptable if they are applied to every pixel in the image and as long as they do not obscure, eliminate, or misrepresent any information present in the original, including the background. Nonlinear adjustments (e.g., changes to gamma settings) must be disclosed in the figure legend.”
The nuts and bolts of this policy are that authors should limit the beautification or touch-up work they apply to an image. Here are the key points:
- Your figure should look like the original capture of the image. Changing portions of the image to make it aesthetically pleasing or to enhance your data improperly is not allowed.
- If an adjustment is absolutely necessary, it must be applied to the entire image and not just a selection.
- Along the same lines, while authors most commonly think of the brightness and contrast tools to tweak a blot or micrograph, resizing should be applied to images with the same care as these other adjustments. If an image size needs to be adjusted, make sure you maintain the aspect ratio. If you stretch or compress an image in just one direction, you are not treating the pixels in the image equally.
- If you make any nonlinear adjustments, such as changes to the gamma setting, you should declare these adjustments in the figure legend. These types of adjustments do not increase or decrease pixel intensity levels evenly across the image. The low and high tones are adjusted at a different rate than the midtones, meaning that, again, not all pixels in the image are treated equally.
- The image background is part of the data and never should be removed or misrepresented in the figure. Remember, background is a hallmark of authentic data.
- Finally, you should be as transparent as possible in the figure and figure legends. Disclosing gel splices, reuse of control data and image acquisition settings allows readers and reviewers properly to assess your data.
Now that you have the rules of the game down, let’s see how well you do with spotting a presentation error. See if you can figure out what each author has done wrong.
These examples, as well as many other types of manipulation, are caught easily by a trained eye and imaging software. Depending on the severity of the manipulation, these alterations could be damaging not only to your reputation but to those of all the co-authors on your paper. Make sure you are preserving the integrity of the scientific record by playing by the rules of the game.
Answers
Example 1
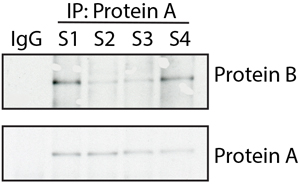
 In this instance, the authors omitted the dividing lines to indicate that different sections of an immunoblot were spliced together to create the final image.
In this instance, the authors omitted the dividing lines to indicate that different sections of an immunoblot were spliced together to create the final image.
Example 2
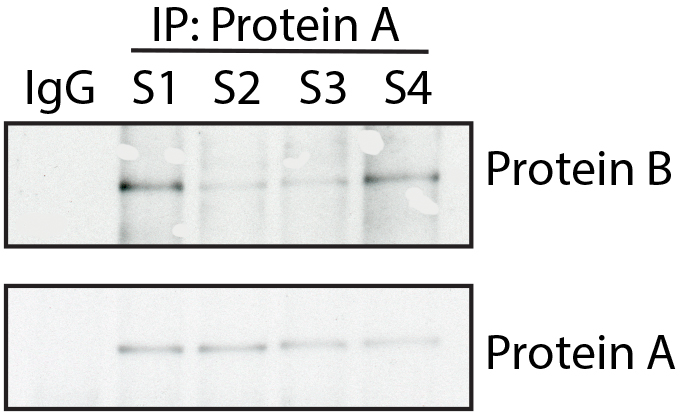 Here, the authors removed some background spots in the immunoblot for Protein B to beautify the image. To show a true representation of the original capture, the authors should have left the spots in the final figure.
Here, the authors removed some background spots in the immunoblot for Protein B to beautify the image. To show a true representation of the original capture, the authors should have left the spots in the final figure.
Example 3
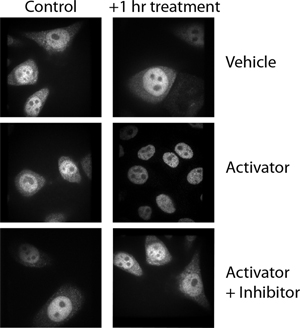
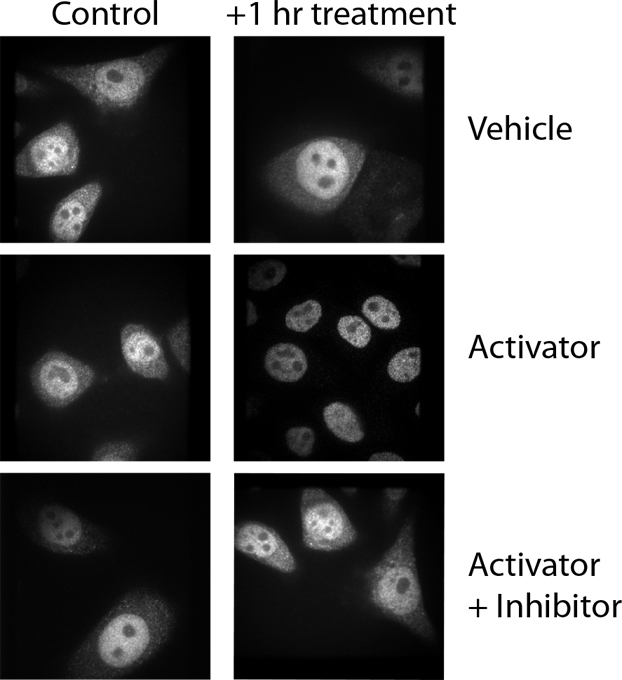 In this example, the authors rotated the control, vehicle-treated panel and inadvertently inserted it into the activator-plus-inhibitor panel. Be sure to label your data effectively so that when you are assembling your figures later, you can determine which image goes with which experimental condition. Simple 1, 2, 3 labels can be hard to decipher a year or even a month after you’ve generated the data. Be sure to check the final figure against the original data to ensure that nothing was switched inadvertently during assembly.
In this example, the authors rotated the control, vehicle-treated panel and inadvertently inserted it into the activator-plus-inhibitor panel. Be sure to label your data effectively so that when you are assembling your figures later, you can determine which image goes with which experimental condition. Simple 1, 2, 3 labels can be hard to decipher a year or even a month after you’ve generated the data. Be sure to check the final figure against the original data to ensure that nothing was switched inadvertently during assembly.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Finding a symphony among complex molecules
MOSAIC scholar Stanna Dorn uses total synthesis to recreate rare bacterial natural products with potential therapeutic applications.

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.