Researchers find a cell surface decorated with sugar-coated RNAs
It’s been a great year for RNA biologists: First the Nobel Prize in chemistry 2020 was awarded to Emmanuelle Charpentier and Jennifer Doudna for the development of CRISPR–Cas9 as a technology for genome editing, and then mRNA vaccines came to our rescue against the COVID-19 pandemic.


What more is there to say about this biomolecule to mark the fourth anniversary of RNA Day? My eureka moment came when I saw a paper in the journal Cell that describes how RNA can be glycosylated and how these sugar-coated RNA molecules, or glycoRNAs, present themselves on a cell’s surface, suggesting they play a role in immune signaling.
Carolyn Bertozzi, a professor of chemistry at Stanford University and the study’s senior author, described the breakthrough: “All of a sudden, you have to rewrite all the textbooks, because we’re dealing with a cell surface that has not just glycoproteins and glycolipids, but also glycoRNA.”
Ryan Flynn, the lead author and an RNA biologist at Harvard University and Boston Children’s Hospital, explained the discovery in simple terms. “If your body is made up of cells, and the cells need to communicate to each other, maybe before this work, the idea was that the cell surface has two hands,” he said. “Our work says that now there are three hands, and we don’t know what that third hand is doing. But there’s a new hand, and it’s out there.”
A bumpy and unexpected start
Flynn joined Bertozzi’s lab as a postdoctoral researcher in 2017, wanting to expand his expertise from RNA to another biopolymer: glycans and carbohydrates. He had an interesting hypothesis regarding the possibility for cytosolic glycosylation of RNA with O-linked beta-N-acetylglucosamine, or O-GlcNAc. He thought this might work because the protein O-GlcNAc transferase, or OGT, responsible for glycosylation, also has an RNA-binding domain.
This is where Bertozzi’s expertise in bio-orthogonal chemistry played an important role. Flynn was using bio-orthogonally functionalized sugars that could be fed to cells to look for sugars attached to RNAs. These functional sugars then could click an affinity probe that allowed him to visualize, enrich and then separate out the labeled molecules.
Flynn set out to look for RNAs that were labeled with the glycans used in that cytosolic glycosylation pathway. He did not find them.
“After nine months of rigorous experiments and controls after controls, I realized that I didn’t know what I had found,” Flynn said. “I asked myself, ‘What do I know about all the experiments I did?’ And I couldn’t come up with any specific conclusions.”
Going through the blots, however, he realized that the only consistency across every cell type tested was the presence of an RNA-sensitive single band that he observed on treatment with N-azidoacetylmannosamine, orManNAz, a functionally labeled precursor for a group of glycans known for their role in glycosylating secretory and cell surface proteins and lipids.
“And that’s where the big question mark and the head scratching started, because in a way ManNAz was a negative control,” Bertozzi said. “There was no reason to believe that RNA could get modified with the kinds of sugars that secreted and cell surface glycoproteins get labeled with, because there’s no framework by which RNA accesses the secretory pathway.”
When Flynn sequenced the RNAs, they all fell into the category of small noncoding RNAs, including types such as transfer, small nuclear and Y RNAs — all structured and highly conserved noncoding RNAs. Researchers do not understand yet the cellular functions of Y RNAs, but they have been identified as autoantigens in a number of autoimmune diseases.
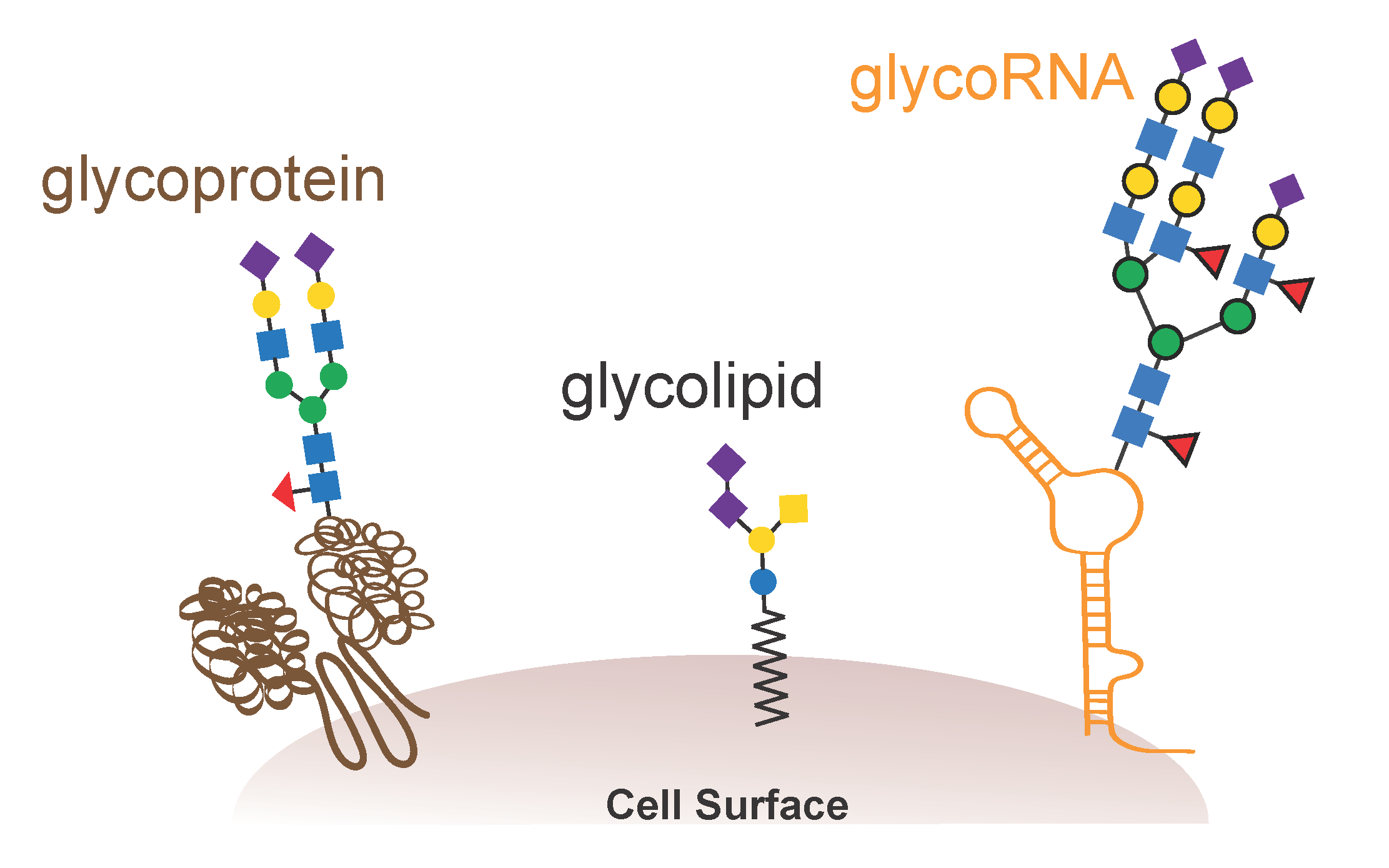
GlycoRNAs, cell surface biology and immune signaling
So where are these RNA present, and what is their function? The team found that the amount of glycoRNA decreases by more than 50% after an hour of incubation with the sialidase, suggesting that more than half of a cell’s glycoRNA is present on its outer membrane.
“I think the position of things often either controls or infers their function,” Flynn said. “And so, if it’s on the cell surface, it really starts to help to fill out the black boxes, and you can begin to design new experiments.”
He noticed that the glycan structures associated with these small RNAs are highly branched and are dominated with sialic acid sugars as capping groups on these branches. This triggered a connection; the Bertozzi lab already was studying a family of sialoglycan binding receptors that are known to be immune modulatory — the Siglec family.
What if glycoRNAs could serve as biological ligands for members of the Siglec family? Flynn tested whether the binding of Siglecs’ receptors to cells was blocked by or inhibited by treatment with RNAse. While most of the Siglecs’ receptor bindings were unaffected, the binding of two, Siglec-11 and Siglec-14, dropped considerably.
“It’s not only that Ryan discovered that there’s this new kind of molecule, but there’s a whole new kind of pathway to produce this kind of molecule that is not understood,” Bertozzi said. “And once again, we are humbled by how little we know about biology.”
What’s next for glycoRNAs?
“It’s funny how things flip,” Bertozzi said. “Early in this project, I was skeptical and wary. I think Ryan was duly skeptical and wary but also intrigued and persistent. And we knew that for us to convince the world that we really had discovered a new molecule was going to be hard. But once the paper got published, I’ve been really amazed at the response to it.”
The ManNAz bio-orthogonal labeling method can’t be applied easily to preserved human tissue samples or patient samples — a major limitation of the study. Scientists will need to develop other glycan labeling strategies to detect natural, unlabeled RNA–glycan conjugates.
Going back to his earlier analogy, Flynn said, “What we know is that the third hand might look like the first two hands but has different properties. Maybe it’s a bigger hand, or it’s stronger or weaker. But there’s a third hand, and it’s able to do something. And so then the question is why do the cells need it? How is it made? Where is it made? How many of the cells have three hands? And does that change in disease? And that’s where we are now.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.
Missing lipid shrinks heart and lowers exercise capacity
Researchers uncovered the essential role of PLAAT1 in maintaining heart cardiolipin, mitochondrial function and energy metabolism, linking this enzyme to exercise capacity and potential cardiovascular disease pathways.

Decoding how bacteria flip host’s molecular switches
Kim Orth will receive the Earl and Thressa Stadtman Distinguished Scientists Award at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

Defining JNKs: Targets for drug discovery
Roger Davis will receive the Bert and Natalie Vallee Award in Biomedical Science at the ASBMB Annual Meeting, March 7–10, just outside of Washington, D.C.

