Starved to death: Can dietary methionine combat cancer?
The organic compounds that come together to form proteins are called amino acids. The human body uses amino acids as sources of energy for functions such as homeostasis, growth, and repair. While the body can produce some amino acids (known as nonessential), others are strictly obtained through food (known as essential).
The essential amino acid methionine, or Met, is critical for genetic regulation, protein production, cell metabolism and DNA repair. Unlike noncancerous cells, most cancer cells cannot recycle Met efficiently; instead, cancer cells rely on a continuous supply of methionine from external sources for growth. This vulnerability is known as Met dependence, or Met stress sensitivity.
Researchers do not know much yet about the mechanisms behind Met dependence in cancer; however, a recent study published in the Journal of Lipid Research has brought us closer to understanding the role of Met dependence in cancer cell lipid metabolism. Peter Kaiser of the University of California, Irvine, and collaborators at the University of California, Davis, Metabolomics Center and University of Texas MD Anderson Cancer Center used Met-dependent and Met-independent breast cancer cell lines to characterize the lipid changes that occur in response to Met-dependent stress.
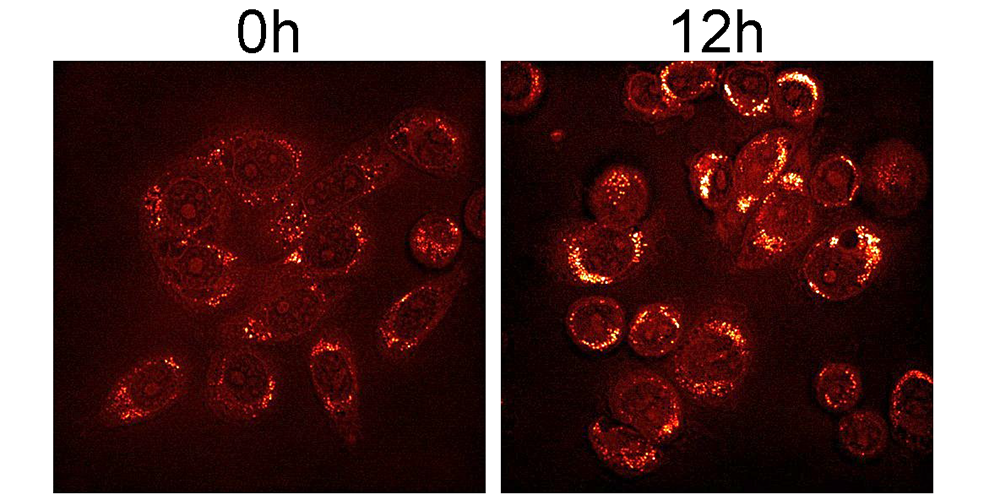
In the cell, diverse lipids make up the cellular membrane and aid in signaling and transport; lipids are also important for nutrient and energy storage. While lipid metabolism is studied widely in relationship to heart disease, researchers know little about lipid metabolism in cancer.
“In cancers, specifically in breast cancer, there has always been a connection to lipid metabolism,” Kaiser said. “We are very interested in understanding how these changes in lipids can affect cancer cells and how they can translate into feasible drug targets.”
Kaiser and colleagues fed cancer cells Met-deficient media to induce stress and then used high-performance liquid chromatography, genetic analysis, and cell microscopy to characterize the changes that occurred in lipids. The researchers found that lipid remodeling and abundance are affected directly by Met-deprivation stress in cancer cells.
Compared to the Met-independent cells (which do not require externally provided Met), the researchers saw an accumulation of lipid droplets, a decrease in lipid synthesis, and a global decrease in all lipid types (except triglycerides; these underwent remodeling), in the Met-dependent cancer cells (which require a continuous external supply of Met). These changes suggest Met stress may affect the endoplasmic reticulum, or ER, an organelle in the cell responsible for many metabolic processes, including lipid synthesis.
“A lot of proteins are folded in the ER,” Kaiser said. “This can lead to a stress response because protein folding becomes impacted in the ER as a consequence of the changes occurring to the lipids.”
These findings support a previous study in which reduced dietary Met helped shrink tumors in rats when used in conjunction with radiation or chemotherapeutics.
Kaiser and his colleagues seek to understand the molecular mechanisms involved in cancer Met dependence. His lab is also interested in the relationship between Met dependence and cell cycle regulation. These studies could increase knowledge of the unique metabolic needs of cancer cells and lead to better therapies.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.
Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

From humble beginnings to unlocking lysosomal secrets
Monther Abu–Remaileh will receive the ASBMB’s 2026 Walter A. Shaw Young Investigator Award in Lipid Research at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

Chemistry meets biology to thwart parasites
Margaret Phillips will receive the Alice and C. C. Wang Award in Molecular Parasitology at the ASBMB Annual Meeting, March 7-10 in Washington, D.C.

ASBMB announces 2026 JBC/Tabor awardees
The seven awardees are first authors of outstanding papers published in 2025 in the Journal of Biological Chemistry.

