From the journals: June/July 2019
We offer a selection of recent papers on a variety of topics from the Journal of Biological Chemistry, the Journal of Lipid Research and Molecular & Cellular Proteomics.
Olive proteins and Parkinson’s disease
Aggregates of alpha synuclein, or alpha SN, are primary components of Lewy bodies, which are linked to the degeneration of dopaminergic neurons in Parkinson’s disease. In the search for therapeutic molecules, researchers have found that phenolic compounds from olive fruit protect against neurodegeneration and exhibit antioxidant properties. Hossein Mohammad–Beigi and an international team screened extracts from 15 olive varieties and found that Koroneiki olives had the most potent effects on alpha SN aggregation. The authors fractionated the extract and identified three compounds that drove alpha SN monomers to form oligomer chains with reduced toxicity. The study was published in the Journal of Biological Chemistry.
Tregs help fight fatty liver disease
Consumed by people around the world, alcohol is a leading cause of illness and mortality. The liver is the organ primarily responsible for alcohol metabolism and can be damaged by excessive or prolonged consumption. This progressive damage leads to alcohol-induced liver disease, or ALD, which presents as a spectrum of symptoms and severities. Fatty livers are the earliest and most common pathology. Recent studies suggest that fat accumulation makes the liver more vulnerable to inflammation, which is further exacerbated when immune cells called T regulatory cells, or Tregs, are deficient.
A recent paper published in the Journal of Lipid Research gives insight into the role Tregs play in ALD. Qin Ning and colleagues from Huazhong University of Science and Technology in China used mice to model alcohol-induced fat accumulation in the liver. They found that diseased mice had depleted Tregs as well as aberrant macrophage activation and cytokine production. Treg transfer to the diseased mice relieved inflammation and lipid metabolic disorder and inhibited macrophage activation and cytokine production. They also found that the effects of Tregs are dependent partially on the cytokine IL-10. Their data show a novel role for Tregs in ALD and highlight a potential therapeutic target.
Viral cue sharpens arthritis drug’s aim
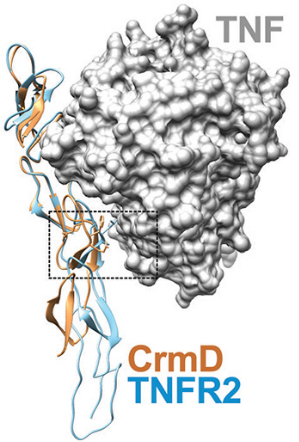 Overlaid crystallographic structures of CrmD in yellow and etanercept in blue bound to TNF. The dashed box surrounds the region of the CrmD that is key to preventing inhibition of lymphotoxin alpha. Pontejo et al.Tumor necrosis factor, or TNF, protein coordinates inflammatory responses after binding to immune cells, but defects can occur in regulatory pathways that lead to overproduction of the protein, causing chronic inflammation and autoimmune disorders. The drug etanercept, marketed as Enbrel, a soluble form of a TNF receptor, has been approved to treat autoimmune disorders such as rheumatoid arthritis. However, it also blocks essential molecules, including lymphotoxin alpha, or LT alpha, thus increasing a patient’s vulnerability to infections, lymphoma and heart failure.
Overlaid crystallographic structures of CrmD in yellow and etanercept in blue bound to TNF. The dashed box surrounds the region of the CrmD that is key to preventing inhibition of lymphotoxin alpha. Pontejo et al.Tumor necrosis factor, or TNF, protein coordinates inflammatory responses after binding to immune cells, but defects can occur in regulatory pathways that lead to overproduction of the protein, causing chronic inflammation and autoimmune disorders. The drug etanercept, marketed as Enbrel, a soluble form of a TNF receptor, has been approved to treat autoimmune disorders such as rheumatoid arthritis. However, it also blocks essential molecules, including lymphotoxin alpha, or LT alpha, thus increasing a patient’s vulnerability to infections, lymphoma and heart failure.
In a study published in the Journal of Biological Chemistry, Sergio Pontejo and colleagues at the Autonomous University of Madrid introduced a component of a poxvirus into etanercept, producing a variant with less than one-sixtieth of the original LT alpha-blocking activity and a mostly retained ability to inhibit TNF proteins.
To evade host immune responses, poxviruses deploy proteins called viral TNF decoy receptors that inhibit native TNF molecules. One of these decoy receptors, CrmD, which is produced by the mousepox-causing ectromelia virus, neutralizes both TNF and LT alpha proteins in mice but does not interfere with LT alpha in humans. The authors of the study sought to understand CrmD’s unique binding ability at a molecular level.
By analyzing how several mutations in CrmD’s binding region affected its binding to TNF and LT alpha, the researchers zeroed in on a motif of three amino acids that prevents the protein from inhibiting human LT alpha. They then mutated etanercept to include this motif and found that the mutation reduced LT alpha-blocking activity more than sixtyfold while weakening TNF inhibition threefold. This safer form of etanercept might replace the traditional form of the TNF inhibitor in clinical use.
— Jonathan Griffin
Counting proteins of a single cell
Computer simulations can help scientists understand complex cell signaling pathways, but, to ensure model accuracy, researchers need parameters determined by experiments with live cells. Akira Komatsubra and colleagues in Japan have established a way to measure protein concentration and dissociation constants at the single-cell level. By attaching EGFP and HaloTag genes to the ends of MAPK1 and RSK2 genes, respectively, the authors enabled fluorescence imaging of proteins encoded by the two modified genes and calculated their concentrations and dissociation constants. The study, published in the Journal of Biological Chemistry, demonstrates an approach to quantifying parameters that could improve the performance of simulation models.
Rethinking a vaccine for malaria
Caused by parasites belonging to the Plasmodium family, malaria is transmitted to humans by female Anopheles mosquitoes. This life-threatening disease is prevalent in developing countries, and the only approved vaccine is largely ineffective, so much recent research has been directed toward developing more potent protection against the parasite. Studies have shown that some attenuated parasite proteins can protect humans, but researchers have been unable to find malaria proteins that confer this protection. Using malaria parasite–infected mice as models, a study by Anthony Siau and colleagues at Nangyang Technological University and Singapore Immunology Network employed immunomic and proteomic techniques to identify a repertoire of parasite proteins associated with protection against Plasmodium yoelii, the parasite used in labs to infect mice. Using such criteria as reproducibility and immunoreactivity, the authors categorized these proteins based on how likely they were to protect against blood-stage malarial infection — the stage when the parasites divide in red blood cells and that is responsible for all clinical malaria symptoms. Their findings were published in the journal Molecular & Cellular Proteomics. When extended to Plasmodium species affecting humans, this extensive data set could be used to help validate and characterize new vaccine formulations for better prevention of malaria.
Tackling an incurable blood cancer
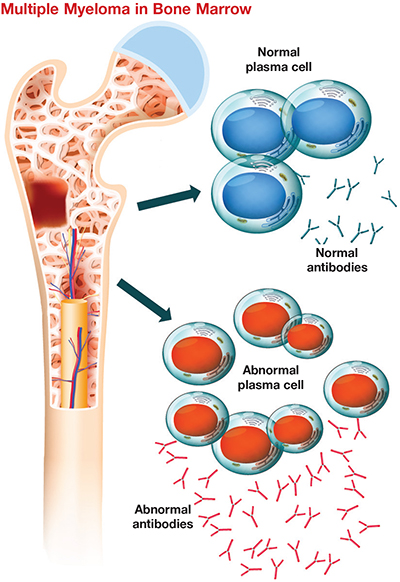 This cartoon shows plasma cells in bone marrow with and without multiple myeloma. Researchers are studying this incurable cancer’s adaptation to lack of oxygen. Clinical Reviews 2018Multiple myeloma, or MM, is a cancer of the plasma cell, a white blood cell responsible for making antibodies, in bone marrow. It is the second most common blood cancer in the U.S. after non-Hodgkin’s lymphoma. MM starts out as a benign condition called monoclonal gammopathy of undetermined significance, or MGUS, and then becomes malignant via an intermediate stage known as smoldering multiple myeloma, or SMM. The lifetime risk of getting MM in the U.S. is less than 1%. The disease’s progression is poorly understood and it has no cure, so recent research has sought to understand the microenvironment that contributes to the pathophysiology of MM.
This cartoon shows plasma cells in bone marrow with and without multiple myeloma. Researchers are studying this incurable cancer’s adaptation to lack of oxygen. Clinical Reviews 2018Multiple myeloma, or MM, is a cancer of the plasma cell, a white blood cell responsible for making antibodies, in bone marrow. It is the second most common blood cancer in the U.S. after non-Hodgkin’s lymphoma. MM starts out as a benign condition called monoclonal gammopathy of undetermined significance, or MGUS, and then becomes malignant via an intermediate stage known as smoldering multiple myeloma, or SMM. The lifetime risk of getting MM in the U.S. is less than 1%. The disease’s progression is poorly understood and it has no cure, so recent research has sought to understand the microenvironment that contributes to the pathophysiology of MM.
A feature of cancer progression is induction of tumor hypoxia as a result of reduced oxygen and nutrient delivery to the tumor cells. MM cells can adapt to hypoxia, which contributes to disease progression. To understand this process better, Astrid Slany and colleagues in Austria and Germany profiled the proteome of primary human MM cells in response to hypoxia induced as the cancer progresses. They analyzed plasma cells from bone marrow of patients with MGUS, SMM and MM and looked for proteins expressed in myeloma cells that aided in their survival, proliferation, and mechanisms to evade apoptosis and immune response. In comparison to nondiseased plasma cells, myeloma cells showed significant changes in the expression of proteins that regulate metabolic processes leading to cell proliferation under hypoxic stress. Also, pro-apoptotic proteins were downregulated, while proteins involved in escaping cell death and immune response were upregulated in hypoxia-stressed MM cells. Their findings were published in the journal Molecular & Cellular Proteomics.
These results indicate that myeloma cells’ adaptation to hypoxia plays a key role in disease progression. Unique proteins in advanced-stage myeloma cells could serve as novel targets for development of improved anti-myeloma treatment strategies.
— Isha Dey
Staph bacteria’s copper resistance
Many hospital surfaces are copper because the metal has antimicrobial properties that can block the spread of disease, but some strains of Staphylococcus aureus have evolved mechanisms to resist copper’s effect. Zuelay Rosario–Cruz of Rutgers University and a team of researchers identified two genes, copB and copL, that enable S. aureus’ copper resistance. Genetic evidence suggested that the CopB protein plays a role in copper export, while nuclear magnetic resonance identified CopL from Bacillus subtilis, a close relative of S. aureus, as a membrane-bound lipoprotein that could bind extracellular copper. These findings, published in the Journal of Biological Chemistry, suggest mechanisms by which S. aureus thwarts copper and could aid in the development of methods to better limit infections.
Reversing mutant accumulation in cancer
The transcription factor p53 functions as a tumor suppressor; in more than half of cancer patients, this protein is mutated and accumulates inside cells. Luciana Rangel and colleagues at the Federal University of Rio de Janeiro report that the small molecule PRIMA-1, which can return mutated p53 to a natural shape, also reverses accumulation of the protein. Their study, published in the Journal of Biological Chemistry, showed that exposing cancer cells to PRIMA-1 reduced aggregates of p53, initiated programmed cell death and inhibited the ability of mutant p53 to trigger aggregation of native p53. The authors suggest these results point toward p53 as a valid drug target.
Digging into the phagosomal proteome
In the process of phagocytosis, immune cells eat up foreign pathogens. The primary phagocytes in the human immune system are dendritic cells, or DCs, which break down pathogens in dynamic compartments called phagosomes and present the resulting antigen to T cells to start an immunogenic response. A phagosome changes its protein composition during its development and in response to various stimuli, but that phagosomal proteome is poorly understood.
In a collaborative study published in Molecular & Cellular Proteomics, Eik Hoffmann and an international team investigated changes in the phagosomal proteome in DCs derived from resting bone marrow versus those stimulated for immune response by lipopolysaccharide, or LPS. Using label-free mass spectrometric analysis, the authors found that upon LPS stimulation, these phagosomes recruit fewer proteins that help them mature and more proteins that help them present the antigen to T cells, thus changing their proteome. The study also identified other proteins previously not known to participate in phagosome function. This preliminary finding paves the way for better understanding of the phagosomal proteome and its dependence on external stimuli.
Lipid signaling may help prevent atherosclerosis
Atherosclerosis is caused by the buildup of fatty plaque deposits in the arteries and can lead to heart attack and stroke. Lysophosphatidic acid, or LPA, is a lipid that functions in signaling in vascular smooth muscle and endothelial cells. While LPA has been shown to be increased in atherosclerotic lesions in mice, the specific roles of LPA and LPA receptors in blood and vascular disease remain an active area of research.
In a recent study published in the Journal of Lipid Research, Susan Smyth of the University of Kentucky and colleagues investigated the role of a specific LPA receptor called LPAR4 in the development of atherosclerosis in mice. They found that while cholesterol levels and lipoprotein distribution were similar in wild-type and Lpar4-knockout atherosclerotic mice, the knockout mice had overall less atherosclerosis and the atherosclerotic lesions that were present were smaller in size.
Previous studies have shown that LPA can promote inflammatory responses, including the switch between M1-type macrophages that are associated with inflammation and M2-type macrophages that are associated with tissue repair and healing. When Smyth and colleagues used LPA to stimulate macrophages isolated from wild-type and Lpar4-knockout mice, they found that macrophages from the knockout mice upregulated a marker associated with M2-type activation. They also detected higher levels of markers indicative of M2-type macrophages in the aortic and coronary arteries from the knockout mice compared to the wild-type mice.
These results suggest that LPAR4 may be involved in recruiting specific immune cell subsets or regulating the phenotypic switch of macrophages from M1- to M2-type, which in turn may promote resolution of plaque inflammation. Furthermore, their research supports the idea that therapeutic development to target LPA pathways may provide an anti-inflammatory approach to prevent some of the complications associated with atherosclerotic disease.
— Courtney Chandler
 Fatty plaque buildup in the heart’s artery causes atherosclerosis, reducing its surface area and hence its oxygen-carrying capacity. Manu5/Wikimedia Commons
Fatty plaque buildup in the heart’s artery causes atherosclerosis, reducing its surface area and hence its oxygen-carrying capacity. Manu5/Wikimedia Commons
Isotope labeling may affect kinetic studies
Kinetic reactions and metabolic pathways can be studied using isotopically labeled substrates. Reactants are labeled by replacing specific atoms with their isotopes and can thus be tracked through time. Serine palmitoyltransferase, or SPT, is the first enzyme in sphingolipid biosynthesis and often is investigated using isotopically labeled L-serine.
In a recent study in the Journal of Lipid Research, Dominic Campopiano of the University of Edinburgh and an international team used SPT from humans and the bacteria Sphingomonas paucimobilis to investigate the effect isotope labels may have on catalysis. SPT is conserved among all organisms capable of producing sphingolipids, yet the eukaryotic isoform is membrane-bound whereas the bacterial isoform is soluble and found in the cytoplasm. Campopiano and colleagues monitored SPT-catalyzed reactions using a series of L-serine substrates that differed in the position of an isotopic label. While the human and bacterial forms of SPT showed similar kinetics in many cases, one substrate revealed a kinetic isotope effect in the bacterial SPT that was absent in the human form. This suggests subtle catalytic differences between cytoplasmic and membrane-bound forms of SPT and that the position of isotope labeling should be considered carefully during kinetic experiments.
Molecular bridges help patch up DNA
Double-strand breaks, or DSBs, in DNA can be lethal to cells, but mechanisms have evolved to correct errors in the genetic code. The protein Ctp1 is key to the initiation of DSB repair, but exactly how it interacts with DNA was until recently unknown. To find out, Sara Andres and colleagues from the National Institute of Environmental Health Sciences and the University of North Carolina performed atomic force microscopy imaging of Ctp1-DNA complexes. The authors write in the Journal of Biological Chemistry that they observed polymerized Ctp1 tetramers on dsDNA, forming bridges between strands; when Ctp1 was mutated, cells were sensitized to DNA damage. These images provide valuable mechanistic insights for studying disease-causing DSB repair defects.
Protons and calcium ions shall not pass
Channelrhodopsins, or ChRs, are light-activated ion channels used in neuroscience to induce activity in neurons. But activating ChRs also can allow passage of protons and calcium ions, which may trigger undesirable responses such as glial acidification or intracellular calcium release. To avoid these effects, Yong Ku Cho and colleagues at the Massachusetts Institute of Technology engineered ChRs that permit the passage of only certain ions. By generating and screening hundreds of ChR2 mutants, they found a combination of mutations that produced a ChR variant with tenfold reductions in calcium and proton flux. Their methods offer a means to customize ChRs and could reveal new principles of optogenetic protein engineering. The study was published in the Journal of Biological Chemistry.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.