JBC: A phospholipid pathway from plants to parasites
Findings by researchers at Washington University in St. Louis may aid in the development of therapies to treat parasitic infections, including malaria, and may help plant scientists one day produce hardier crops. The research team’s work was published in the Journal of Biological Chemistry.
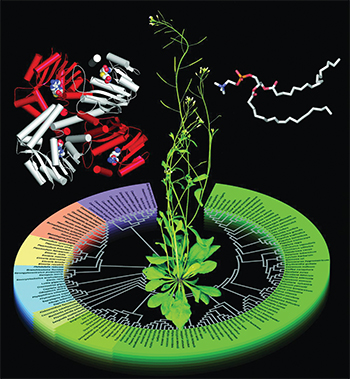 A study explains how structures of Arabidopsis phosphoethanolamine methyltransferase, or PMT, (left) are evolutionarily related to PMT sequences from different organisms. Phosphatidylcholine (right) is PMT's product. Courtesy of Soon Goo Lee and Joseph JezCholine is an essential nutrient that we get from certain foods, including eggs, meat, leafy greens and nuts. The human body converts choline into phosphocholine, or pCho, which it in turn converts into (among other essential building blocks) phosphatidylcholine, or PtdCho, a component of cell membranes. Plants can’t acquire the nutrient from the environment and so must synthesize pCho from scratch. The biochemical pathway plants use to synthesize pCho also is found in nematodes and the malaria parasite Plasmodium.
A study explains how structures of Arabidopsis phosphoethanolamine methyltransferase, or PMT, (left) are evolutionarily related to PMT sequences from different organisms. Phosphatidylcholine (right) is PMT's product. Courtesy of Soon Goo Lee and Joseph JezCholine is an essential nutrient that we get from certain foods, including eggs, meat, leafy greens and nuts. The human body converts choline into phosphocholine, or pCho, which it in turn converts into (among other essential building blocks) phosphatidylcholine, or PtdCho, a component of cell membranes. Plants can’t acquire the nutrient from the environment and so must synthesize pCho from scratch. The biochemical pathway plants use to synthesize pCho also is found in nematodes and the malaria parasite Plasmodium.
In plants, the enzymatic reaction that produces pCho is essential for normal function and for responding to stresses. Plant pCho is converted into PtdCho, which builds membranes that can adjust their rigidity in response to temperature changes. pCho also gets converted into molecules that help plants survive high salt. The enzymes that produce plant pCho are called phosphoethanolamine methyltransferases, or PMTs.
Soon Goo Lee, a postdoctoral research fellow at Washington University in the lab of Joseph Jez (a JBC associate editor), has been fascinated by PMTs in both plants and parasites for many years.
“Understanding the PMT enzyme is key to engineer plants with improved stress tolerance and enhanced nutrients,” Lee said.
Since the PMT-catalyzed pathway is found in parasites but not humans, Lee and Jez’s team is looking for inhibitors of this enzyme to treat diseases caused by these parasites.
The new study explains that PMTs of the model plant Arabidopsis thaliana share core features of PMTs from parasites, with almost identical structure at the active site. But the plant PMTs are roughly twice as large as the parasite ones, with large sections that can rearrange themselves to carry out multiple chemical reactions.
The three PMT types in the plant — which were thought to carry out the same function — actually appear to play different roles depending on where they are found in the plant. Plant growth experiments showed that one type of PMT was essential for root development and salt tolerance, whereas the other two had no effect on roots and instead seemed to be found primarily in leaves.
In the long run, this big-picture view of PMTs in different organisms offers routes to engineer enzymes with different functions. “I love these kinds of stories,” Lee said, “where I can look from the atomic (structure) to the physiological level to explain why these enzymes have different forms and how they work.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.

Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.

Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.

Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)