Metal art, microbial culture
Bacteria and fungi are in many ways the enemies of art and culture — mold destroys books and is threatening the Lascaux cave paintings, biofilms form unattractive scum on roofs and sculptures, and many microbes cause wear and tear on metal and stone. But just as biomedical research on the microbiome is uncovering the many benefits bacteria can provide to human health, some in the field of conservation and restoration science hope that certain bacteria could be used to reverse the deterioration of cultural heritage objects.
 A statue of Osiris from the collections of the Musée d’Ethnographie Neuchâtel, Switzerland, treated with a bio-passivation method to stabilize its corrosive layer.Courtesy of Emmanuelle Domon Beuret, Laténium archaeological museum
A statue of Osiris from the collections of the Musée d’Ethnographie Neuchâtel, Switzerland, treated with a bio-passivation method to stabilize its corrosive layer.Courtesy of Emmanuelle Domon Beuret, Laténium archaeological museum
Pilar Junier, a professor at the University of Neuchâtel in Switzerland, has spent a lot of time thinking about what bacteria can do. “Every reaction in biology has two sides, like a coin,” she said. “One side is deleterious, but the other can be beneficial, if you change the context.”
Junier is an environmental microbiologist who has studied microbial activities in lakes and sediments as well as in applied contexts like bioremediation of uranium. She began to collaborate in 2013 with Edith Joseph, a materials chemist with a background in art conservation, who thought that the chemical transformations needed to preserve some objects might be carried more effectively out by living organisms.
Microbes “are the best chemists in the world,” Junier said. “Why don’t we use them?”
Microbes to the rescue
Edith Joseph started her research in 2006, using microorganisms for the preservation of heritage materials in Italy, and arrived in Switzerland in 2010 to work on this subject at the Swiss National Museum. Today she works at the University of Neuchâtel, collaborating with Junier, as well as in the conservation-restoration department of the nearby Haute Ecole Arc.
Joseph’s first project used fungi to preserve copper-containing objects.She was interested in corrosion both of large outdoor objects — monuments, sculptures and architectural features subjected to pollution or salty sea air — and of fragile archaeological artifacts. When the copper in these objects comes into contact with corrosive agents such as chloride ions, irreversible decay sometimes called “bronze disease” occurs. Outdoor monuments thus afflicted can leach copper into the environment; small items can flake, crack and fall apart. If items cannot be stabilized by placing them in an environment with controlled humidity and temperature, they often are treated with resins or waxes or with industrial corrosion inhibitors like benzotriazole.
 Sculptures undergoing microbial bio-passivation treatment before being displayed in the sculpture park Légende d’Automne, Lausanne, Switzerland. These sculptures are part of an accessible art project and are intended to be touched by the blind and visually impaired. courtesy of Edith Joseph, University of Neuchâtel
Sculptures undergoing microbial bio-passivation treatment before being displayed in the sculpture park Légende d’Automne, Lausanne, Switzerland. These sculptures are part of an accessible art project and are intended to be touched by the blind and visually impaired. courtesy of Edith Joseph, University of Neuchâtel
However, if the copper hydroxychlorides on the object’s surface are transformed into a nonreactive “passive” patina, the object could be protected. One such patina is copper oxalate. “Copper oxalate forms a kind of protective layer,” Joseph said. “It’s passivating the surface (to avoid) any exchange of ions with the copper and water, so you have no over-corrosion.”
As it happens, oxalate is produced widely in the fungal kingdom as a defense against insects; oxalic acid-producing fungi sometimes are used as insecticides in organic agriculture. Furthermore, fungi growing in copper-polluted areas produce oxalate to chelate copper ions and protect themselves from copper toxicity.
After several years of research on a copper-resistant strain of the insecticidal fungus Beauvaria bassiana — isolated from a vineyard in which copper had been used for years as a fungicide — Joseph and her colleagues developed a ready-to-use kit called Biopatina for conservator–restorers. The kit consists of the fungus and a growth medium; the two are mixed together, spread on the surface of the copper object, and allowed to incubate for several days or weeks while the fungus produces copper oxalate. Then the fungus is washed off. Joseph has led workshops on the use of Biopatina in Switzerland and France, focusing on outdoor sculptures, and has sent samples of it to conservator-restorers in Europe and Canada.
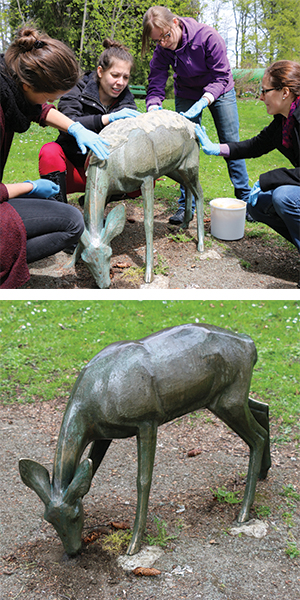 Above: Students from the Haute Ecole Arc Conservation-Restauration use the Biopatina bio-passivation method on a contemporary sculpture in the Park Gallet, la Chaux-de-Fonds, Switzerland.
Above: Students from the Haute Ecole Arc Conservation-Restauration use the Biopatina bio-passivation method on a contemporary sculpture in the Park Gallet, la Chaux-de-Fonds, Switzerland.Below: The sculpture after treatment.courtesy of dith Joseph, Haute Ecole Arc Conservation-Restauration.
Myriam Krieg, a conservator–restorer at the Aventicum Site et Musée romains d’ Avenches, in the Swiss Midlands canton of Vaud, works mostly on Roman artifacts, although some objects from the Celtic Iron Age also have been found recently in the area. Krieg participated in Biopatina workshops in 2015 and 2016, where a team of conservators practiced using the technique on contemporary sculptures.
“Optically, I think the treatment produces a natural, less shiny surface, in contrast to what we see with other products we use,” she said. “I like also the fact that we induce the formation of copper minerals, and we are not dealing with synthetic (resins or coatings) that are really strangers to the object.”
Krieg hopes to soon begin using the Biopatina kit on copper alloy artifacts such as coins or fibulae (Roman or Iron Age brooches) in her work.
Mining microbial resources
Whereas the copper-chelating fungi were isolated from vineyards, Joseph’s team looked close to home in the sediments of Lake Neuchâtel for bacteria that could be used in projects involving iron. Under current conservation practice, to remove chloride ions, highly corroded iron objects must be soaked in alkaline sulfide baths in a time-consuming process.
The iron conservation project is at a much earlier stage than the work with copper. The team is carrying out tests first on iron ions in solution, then on solid iron compounds synthesized in the lab, and then on iron offcuts from foundries, experimentally corroded at the French Corrosion Institute in Brest. Recently, they moved on to experiments with Roman nails and other small objects that museum staff has determined can be sacrificed, and they eventually will turn to more valuable artifacts and the iron architectural features of an outdoor music pavilion in Neuchâtel.
The team still is trying to identify iron-reducing bacteria that could convert rust into iron phosphate or iron carbonate and are sufficiently easy to work with. Iron-reducing reactions occur under only anaerobic conditions, which would be difficult to set up in an art-conservation lab; the team therefore plans to identify bacterial species that can grow in the presence of oxygen but then can be applied in a thick gel with minimal oxygen penetration. One bacterial candidate turned out to be a potential fish pathogen, making it problematic to grow in large batch cultures that could be released accidentally into the environment. Another bacterium could perform the needed reactions but only in culture media with high salt concentrations, which would exacerbate the very problem the bacteria are intended to solve.
Although genetic engineering potentially could solve some of these problems, strict regulation of genetically modified organisms in Europe and negative public perceptions of GMOs mean the team is not pursuing that route. Instead, they continue to look for microbes with the right combination of traits in natural environments.
 Edith Joseph is a researcher at the University of Neuchatel and the Haute Ecole Arc who is developing methods for using microbes to preserve art. Edith Joseph is a researcher at the University of Neuchatel and the Haute Ecole Arc who is developing methods for using microbes to preserve art. |
 Pilar Junier is a microbiologist at the University of Neuchatel who collaborates with Edith Joseph on microbial conservation projects. Pilar Junier is a microbiologist at the University of Neuchatel who collaborates with Edith Joseph on microbial conservation projects. |
 Myriam Krieg is a conservator-restorer at the Site et Musee Romains d’ Avenches in Switzerland. Myriam Krieg is a conservator-restorer at the Site et Musee Romains d’ Avenches in Switzerland. |
“(We) try to see, ‘What are the conditions that we need? What are the potential contaminants in the system that could poison the metabolism?’ to try to identify the very organisms that can tolerate those conditions,” Junier said. “We always say: ‘What do you want to convert? Is it a redox reaction? Is it simply absorption? Is it passivation? What do you want to do chemically speaking?’ And then based on that, you say, ‘Which are the microbial metabolisms that fit these reactions?’”
These investigations into the capabilities of natural isolates can serendipitously lead to new avenues of research in environmental chemistry and microbiology. For example, iron reduction in bacteria has been studied mainly in the model organisms Shewanella and Geobacter. But the iron reducers the team isolated from lake sediments were from the genus Aeromonas, which is not known to have the cytochrome proteins used in iron reduction reactions in the model species. Aeromonas species are common in aquatic sediments, but it’s unknown whether they contribute to metal cycling in aquatic ecosystems.
 Robert J. Koestler is the director of the Museum Conservation Institute at the Smithsonian Institution.
Robert J. Koestler is the director of the Museum Conservation Institute at the Smithsonian Institution.
Very little is known about iron reduction in Aeromonas, Junier said. “So we are trying to pinpoint the mechanism and try to see how much Aeromonas participates in the iron cycle in sediments.”
Two sides to every coin
Once the right bacteria are identified, successfully deploying them in the real world presents its own challenges. Biotechnological applications always have issues of scale and cost-effectiveness as well as being subject to unpredictable variables when they move out of the lab. There are also issues unique to art and culture.
Robert J. Koestler, the director of the Museum Conservation Institute at the Smithsonian Institution, is familiar with the challenges.
“Several groups in Europe in the 1990s tried to harness bacteria to produce calcium carbonate to repair deteriorated marble or limestone historic buildings,” Koestler said. “Initial results seemed promising, but the process is no longer in vogue. This may be because the calcium carbonate formed by the bacteria did not adhere well to the marble or limestone … or because the process itself was spotty — you cannot get microbes to grow evenly across a surface.”
Occupational hazards of art preservation
Edith Joseph pursued microbial methods of treating heritage objects in part because she shares a concern about the occupational and environmental health hazards of standard conservation techniques, which often involve toxic solvents and chemical mixtures.
The U.S. Environmental Protection Agency considers benzotriazole, an industrial corrosion inhibitor often used by conservators to treat bronze artworks, to be a “contaminant of emerging concern” for environmental and human health. On the other hand, if outdoor copper objects are not treated against corrosion, the copper itself is a pollutant if it leaches into the environment.
“At the beginning, the idea was mostly about operator risk, for (conservators) to use safe treatments,” Joseph said. “But now, I always point out if there’s too much copper in the water, then it’s also not good for the environment.”
Conservation professionals who were concerned about occupational and environmental safety and sustainability created a new academic conference, called Green Conservation of Cultural Heritage, which now has been held twice in Italy. Joseph has presented at this conference, pitching her team’s treatments as safer alternatives for workers and the environment.
Myriam Krieg, the conservator–restorer in Avenches, is not acutely concerned about safety issues in her line of work, because operators use personal protective equipment and facilities designed for chemical safety, but she welcomes the development of biologically based alternatives.
“If we have to, we can deal with (toxic chemicals),” Krieg said. “But if we have the choice, if we have an alternative, we will opt for a less toxic treatment.”
There is a further ethical consideration, Koestler said, because chemically altering objects, whether by conventional chemical treatments or using microbes, necessarily destroys information about them. If the original corrosion layer is removed, information about the circumstances under which it corroded and weathered is lost. This makes some types of historical analyses impossible and can make it more difficult to spot fakes by obscuring artificial corrosion introduced by counterfeiters.
“If you can avoid introducing foreign substances, I would not do it,” said Krieg, the conservator–restorer at Avenches. “The best thing is to have the least impact.” If a treatment is unavoidable, taking a sample before intervention can make it possible to further analyze the original material, she said. “Often it’s a compromise: If you do nothing, you might lose the whole object.”
The compromises between conflicting conservation needs, and between the conflicting needs and abilities of microbes and humans, are evident in the example of the earliest-stage project in progress in Joseph’s lab: marine archaeological wood conservation. Magdalena Albelda Berenguer, a Ph.D. student in the lab, is working on solving a widespread problem that plagues wood rescued from shipwrecks.
Sunken wooden ships are typically well-preserved in the anoxic conditions in the deep sea. But while they are resting on the sea floor, a series of reactions occur: Iron ions from corroding iron parts of the ship react with sulfur produced by sulfate-reducing marine microorganisms; the resulting iron sulfides permeate the waterlogged wood. When this wood is taken out of the water and exposed to oxygen, acids and sulfate salts form, breaking down the wood.
The problem has plagued the 17th century Swedish warship Vasa, which was recovered fully intact from a harbor near Stockholm in the 1960s and is on display at a popular museum there. Conservators must maintain the ship continuously, attempting to remove iron or raise the pH with applications of ammonia, and research is ongoing to find a long-term solution for the weakening wood.
 Magdalena Albelda Berenguer is a Ph.D. student in Edith Joseph’s laboratory, working on microbial preservation of marine archaeological wood.
Magdalena Albelda Berenguer is a Ph.D. student in Edith Joseph’s laboratory, working on microbial preservation of marine archaeological wood.Albelda hopes to identify bacteria that can remove iron sulfides from future marine wood finds to pre-empt the problem. But wood, as an organic substance, can be consumed by bacteria, introducing the risk of seriously damaging it.
“The most difficult part to start with is to see which microorganism (to use), and to always think how that could be applied later on,” Albelda said.
Many bacterial candidates are unsuitable because they cannot risk being used on valuable objects that can’t be damaged.
But Albelda echoes her colleagues when she talks about the potential for harnessing bacteria for conserving cultural heritage. Conservation and microbiology, she said, “are very separated worlds, but they have a lot of things in common, because a lot of the deterioration in the objects is made by microorganisms.”
“In the end, everything is nature,” Edith Joseph said. “But it’s nice to see that nature can help us to preserve something for the next generation, and we are not alone in front of this problem.”
 The 17th century wooden warship the Vasa, viewed here from the bow, sank on its maiden voyage and was salvaged in 1961. It is now on display at the Vasa Museum in Stockholm.Courtesy of Javier Kohen/Wikimedia Commons
The 17th century wooden warship the Vasa, viewed here from the bow, sank on its maiden voyage and was salvaged in 1961. It is now on display at the Vasa Museum in Stockholm.Courtesy of Javier Kohen/Wikimedia Commons
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

E-cigarettes drive irreversible lung damage via free radicals
E-cigarettes are often thought to be safer because they lack many of the carcinogens found in tobacco cigarettes. However, scientists recently found that exposure to e-cigarette vapor can cause severe, irreversible lung damage.

Using DNA barcodes to capture local biodiversity
Undergraduate at the University of California, Santa Barbara, leads citizen science initiative to engage the public in DNA barcoding to catalog local biodiversity, fostering community involvement in science.

Targeting Toxoplasma parasites and their protein accomplices
Researchers identify that a Toxoplasma gondii enzyme drives parasite's survival. Read more about this recent study from the Journal of Lipid Research.

Scavenger protein receptor aids the transport of lipoproteins
Scientists elucidated how two major splice variants of scavenger receptors affect cellular localization in endothelial cells. Read more about this recent study from the Journal of Lipid Research.

Fat cells are a culprit in osteoporosis
Scientists reveal that lipid transfer from bone marrow adipocytes to osteoblasts impairs bone formation by downregulating osteogenic proteins and inducing ferroptosis. Read more about this recent study from the Journal of Lipid Research.

Unraveling oncogenesis: What makes cancer tick?
Learn about the ASBMB 2025 symposium on oncogenic hubs: chromatin regulatory and transcriptional complexes in cancer.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)