Study suggests that estrogen may drive nicotine addiction in women
A newly discovered feedback loop involving estrogen may explain why women might become dependent on nicotine more quickly and with less nicotine exposure than men. The research could lead to new treatments for women who are having trouble quitting nicotine-containing products such as cigarettes.
Sally Pauss is a doctoral student at the University of Kentucky College of Medicine in Lexington. She led the project.

“Studies show that women have a higher propensity to develop addiction to nicotine than men and are less successful at quitting,” said Pauss, who is working under the supervision of Terry D. Hinds Jr., an associate professor. “Our work aims to understand what makes women more susceptible to nicotine use disorder to reduce the gender disparity in treating nicotine addiction.”
The researchers found that the sex hormone estrogen induces the expression of olfactomedins, proteins that are suppressed by nicotine in key areas of the brain involved in reward and addiction. The findings suggest that estrogen–nicotine–olfactomedin interactions could be targeted with therapies to help control nicotine consumption.
Pauss will present the research at Discover BMB, the annual meeting of the American Society for Biochemistry and Molecular Biology, which will be held March 23–26 in San Antonio.
“Our research has the potential to better the lives and health of women struggling with substance use,” she said. “If we can confirm that estrogen drives nicotine seeking and consumption through olfactomedins, we can design drugs that might block that effect by targeting the altered pathways. These drugs would hopefully make it easier for women to quit nicotine.”
For the new study, the researchers used large sequencing datasets of estrogen-induced genes to identify genes that are expressed in the brain and exhibit a hormone function. They found just one class of genes that met these criteria: those coding for olfactomedins. They then performed a series of studies with human uterine cells and rats to better understand the interactions between olfactomedins, estrogen and nicotine. The results suggested that estrogen activation of olfactomedins — which is suppressed when nicotine is present — might serve as a feedback loop for driving nicotine addiction processes by activating areas of the brain’s reward circuitry such as the nucleus accumbens.
The researchers are now working to replicate their findings and definitively determine the role of estrogen. This knowledge could be useful for those taking estrogen in the form of oral contraceptives or hormone replacement therapy, which might increase the risk of developing a nicotine use disorder.
The investigators also want to determine the exact olfactomedin-regulated signaling pathways that drive nicotine consumption and plan to conduct behavioral animal studies to find out how manipulation of the feedback loop affects nicotine consumption.
Sally Pauss will present this research during a poster session from 4:30–6:30 p.m. CDT on Monday, March 25, in the exhibit hall of the Henry B. González Convention Center (Poster Board No. 152) (abstract).
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
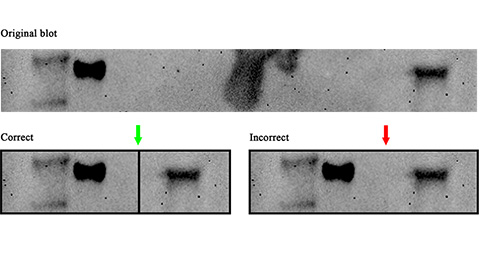
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.
Key regulator of cholesterol protects against Alzheimer’s disease
A new study identifies oxysterol-binding protein-related protein 6 as a central controller of brain cholesterol balance, with protective effects against Alzheimer’s-related neurodegeneration.

