The remaining frontiers in fighting hepatitis C
A, B, C, D, E: It’s a short, menacing alphabet representing the five types of virus causing viral hepatitis, a sickness afflicting some 400 million people around the world today.
This story was originally published by Knowable Magazine.
Hepatitis viruses are a set of very different pathogens that kill 1.4 million people annually and infect more than HIV and the malaria pathogen do combined. Most of the deaths are from cirrhosis of the liver or hepatic cancer due to chronic infections with hepatitis viruses B or C, picked up through contact with contaminated blood.
Hepatitis B was the first of the five to be discovered, in the 1960s, by biochemist Baruch S. Blumberg. Hepatitis A, which is most commonly spread through contaminated food and water, was next, discovered in 1973 by researchers Stephen Mark Feinstone, Albert Kapikian and Robert Purcell.

Screening tests for those two types of viruses paved the way to discovering a third. In the 1970s, hematologist Harvey Alter examined unexplained cases of hepatitis in patients after blood transfusions and found that only 25 percent of such cases were caused by the hepatitis B virus, and none were linked to the hepatitis A virus. The rest were caused by an unidentified transmissible agent that could persist in the body as a chronic infection and lead to liver cirrhosis and liver cancer.
The agent behind this disease, named non-A, non-B hepatitis, remained a mystery for a decade until Michael Houghton, a microbiologist working at the biotechnology company Chiron Corporation, and his team sequenced the agent’s genome in 1989 after years of intensive investigation. They identified it as a novel virus of the family to which yellow fever virus belongs: the flaviviruses, a group of RNA viruses often transmitted through the bite of infected arthropods.
But there was more to the story. Scientists needed to show that this new virus could, indeed, cause hepatitis C on its own — a feat achieved in 1997, when Charles M. Rice, then a virologist at Washington University in St. Louis, and others succeeded in creating a form of the virus in the lab that could replicate in the only animal model for hepatitis C, the chimpanzee. When they injected the virus into the liver of chimpanzees, it triggered clinical hepatitis, demonstrating the direct connection between hepatitis C and non-A, non-B hepatitis.
The findings led to lifesaving hepatitis C tests to avert infections through transfusions with contaminated blood, as well as for the development of effective antiviral medications to treat the disease. In 2020, in the thick of the SARS-CoV-2 pandemic, Alter, Houghton and Rice received a Nobel Prize in Medicine for their work on identifying the virus.
To learn more about hepatitis C history and the treatment and prevention challenges that remain, Knowable Magazine spoke with Rice, now at the Rockefeller University, at the 72nd Lindau Nobel Laureate Meeting in Germany in June 2023. This conversation has been edited for length and clarity.
What were the challenges at the time you began your research on hepatitis C?
The realization that an agent was behind non-A, non-B hepatitis had initiated a virus hunt to try and figure out what the causative agent was. Michael Houghton and his group at Chiron won that race and reported the partial sequence of the virus in 1989 in Science.
It was an interesting kind of a dilemma for me as an early-stage assistant professor at Washington University in St. Louis, where I’d been working on yellow fever. All of a sudden, we had this new human virus that dropped into our laps and joined the flavivirus family; we had to decide if we were going to shift some of our attention to work on this virus. Initially, people in the viral hepatitis field invited us to meetings, but because we were doing work on the related virus, yellow fever, not because we were considered majors player in the field.
The main challenge was that we could not grow the virus in cell culture. And the only experimental model was the chimpanzee, so it was really difficult for laboratories to study this virus.
There were two major goals. One was to establish a cell culture system where you could replicate the virus and study it. And the other was try and create a system where we could do genetics on the virus. It was shown to be an RNA virus, and the collection of tools available for modifying RNA at that time, in the early 1990s, was not the same as it was for DNA. Now that’s changed to some extent, with modern editing technologies.
If there’s one lesson to be learned from this hepatitis C story, it’s that persistence pays off.

This journey started with an unknown virus and ended up with treatment in a relatively short period of time.
I don’t think it was a short period of time, between all of the failures to actually get a cell culture system and to show that we had a functional clone. From 1989, when the virus sequence was reported, to 2011, when the first antiviral compounds were produced, was 22 years.
And then, that initial generation of treatment compounds was not the greatest, and they were combined with the treatment that we were trying to get rid of — interferon — that made people quite ill and didn’t always cure them. They only had about a 50 percent cure rate.
It was 2014 when the interferon-free cocktails came about. And that was really amazing.
There were people who thought, “You are not going to be able to develop a drug cocktail that can eliminate this virus.” It was presumptuous to think that one could, but it was accomplished by biotech and the pharmaceutical industry. So it is really quite a success story, but I wish it could have been faster.

What are the current challenges in combating hepatitis C?
One thing that was a little sobering and disappointing for me was that when these medical advances are made and shown to be efficacious, it is not possible to get these drugs to everybody who needs them and successfully treat them. It’s a lot more complicated, in part because of the economics — how much the companies decide to charge for the drugs.
Also, it’s difficult to identify people who are infected with hepatitis C, because it’s often asymptomatic. Even when identified, getting people into treatment is challenging given differences in public health capabilities which vary at the local, national and global levels. So we have wonderful drugs that can basically cure anybody, but I think we still could use a vaccine for hepatitis C.
During the first year of the Covid-19 pandemic, you won the Nobel Prize for the discovery of the hepatitis C virus. What was that experience like?
It was December 2020, and we were working on SARS-Cov-2 in the peak of the pandemic in New York City. My spouse and the dogs were off at our house in Connecticut, and I was living in the apartment in Manhattan. And I got this call at 4:30 in the morning. It was pretty shocking.
The pandemic made people more aware of what a highly infectious, disease-causing virus can do to our world. It encouraged the rapid dissemination of research results and more open publications. It also really made us appreciate how the same virus does different things depending upon who’s infected: In the case of Covid-19, it’s not good to be old, for example.
After many decades working with viruses, what would you say is the next frontier in virology?
There’s a lot that we don’t understand about these viruses. The more we study them, the more we understand about ourselves, our cells and our antiviral defense systems.
And there’s also great power in terms of being able to diagnose new viruses. The sequencing technology, the functional genomics technologies, all of those things, when applied to virology, give us a much richer picture of how these viruses interact with cells. I think it’s a golden age.

You have been working with flaviviruses (dengue, Zika, yellow fever and hepatitis C) for many decades. Zika and dengue pose an ongoing threat worldwide and, in particular, Latin America. Based on the successful example of hepatitis C, what can scientific research do to mitigate the impact of these viruses?
For viruses like Zika, developing a vaccine is probably going to be fairly straightforward — except that since Zika is so transient, it makes it hard to prove that your vaccine works. You would have to do a human challenge study, in which volunteers are deliberately exposed to an infection in a safe way with health-care support.
For dengue, it’s much more difficult, because there are four different serotypes — different versions of the same virus — and infection with one serotype can put you at increased risk of more severe disease if you get infected with a second serotype. Eliciting a balanced response that would protect you against all four dengue serotypes is the holy grail of trying to develop a dengue vaccine.
People are using various approaches to accomplish that. The classic one is to take live attenuated versions — weakened forms of viruses that have been modified so they can’t cause severe illness but can still stimulate the immune system — of each of the four serotypes and mix them together. Another is to make chimeric viruses: a combination of genetic material from different viruses, resulting in new viruses that have features of each of the four dengue serotypes, engineered into the backbone of the yellow fever vaccine. But this hasn’t worked as well as people have hoped. I think the cocktail of live, attenuated dengue variants is probably the most advanced approach. But I would guess that given the success of Covid-19 mRNA vaccines, the mRNA approach will also be tried out.
These diseases are not going to go away. You can’t eradicate every mosquito. And you can’t really immunize every susceptible vertebrate host. So occasionally there’s going to be spillover into the human population. We need to keep working on these because they are big problems.
You began your career at the California Institute of Technology studying RNA viruses, such as the mosquito-borne Sindbis virus, and then flaviviruses that cause encephalitis, polyarthritis, yellow fever and dengue fever. Later on, you also studied hepatitis C virus. Is there any advantage for virologists in changing the viruses they study throughout their career?
They’re all interesting, right? And they are all different in their own ways. I say that my career has been a downward spiral of tackling increasingly intricate viruses. Initially, the alphaviruses — a viral family which includes chikungunya virus, for example — were easy. The classical flaviviruses — like yellow fever, dengue fever, West Nile viruses and Zika virus, among others — were a little more difficult, but the hepatitis C virus was impossible for 15 years, until we, and others, finally achieved a complete replication system in the laboratory.
We coexist daily with viruses, but the pandemic may have given people the idea that all these microorganisms are invariably life-threatening.
We have to treat them with respect. We’ve seen what can happen with the emergence of a novel coronavirus that can spread during an asymptomatic phase of infection. You can’t be prepared for everything, but in some respects our response was a lot slower and less effective than it could have been.
If there’s anything that we’ve learned over the last 10 years with the new nucleic acid sequencing technologies, it’s that our past view of the virosphere was a very narrow. And if you really look at what’s out there, the estimated virus diversity is a staggering number, like 1031 types. Although most of them are not pathogenic to humans, some are. We have to take this threat seriously.
Is science prepared?
I think so, but there has to be an investment, a societal investment. And that investment has to not only be an investment in infrastructure that can react quickly to something new, but also to establish a repository of protective antibodies and small molecules against viruses that we know could be future threats.
Often, these things go in cycles. There’s a disaster, like the Covid-19 pandemic, people are changed by the experience, but then they think “Oh, well, the virus has faded into the background, the threat is over.” And that’s just not the case. We need a more sustained plan rather than a reactive stance. And that’s hard to do when resources and money are limited.
What is the effect of science illiteracy, conspiracy theories and lack of science information on the battle against viruses?
These are huge issues, and I don’t know the best way to combat them and educate people. Any combative, confrontational kind of response — it’s just not going to work. People will get more resolute in their entrenched beliefs and not hear or believe compelling evidence to the contrary.
It’s frustrating. I think that we have amazing tools and the power to make really significant advances to help people. It is more than a little discouraging for scientists when there’s a substantial fraction of people who don’t believe in things that are well-supported by facts.
It’s in large part an educational problem. I think we don’t put enough money into education, particularly early education. A lot of people don’t understand how much of what we take for granted today is underpinned by science. All this technology — good, bad or ugly — is all science.
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.
Read more
The cellular conundrums of hepatitis C: After Charles Rice shared the 2020 Nobel Prize in physiology or medicine, he talked to ASBMB Today about his career unraveling the genomic secrets of flaviviruses and other positive-strand RNA viruses.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
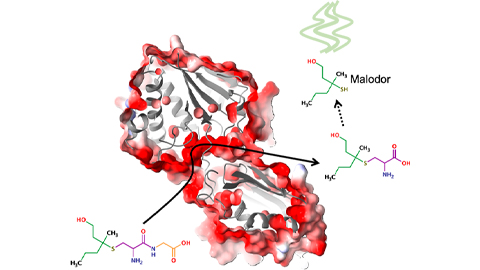
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.
Proteomic variation in heart tissues
By tracking protein changes in stem cell–derived heart cells, researchers from Cedars-Sinai uncovered surprising diversity — including a potential new cell type — that could reshape how we study and treat heart disease.

Parsing plant pigment pathways
Erich Grotewold of Michigan State University, an ASBMB Breakthroughs speaker, discusses his work on the genetic regulation of flavonoid biosynthesis.

