From the journals: JBC
The timing of lipid metabolism. Modifying actin assembly dynamics. How Lyme disease evades the immune system. Read about papers on these topics recently published in the Journal of Biological Chemistry.
The timing of lipid metabolism

The liver is a hub of lipid metabolism, with hepatocytes regulating uptake, esterification, oxidation and secretion of fatty acids and lipid droplet storage. Disruption of the molecular clock (a transcription- and translation-based feedback loop) or metabolic/redox oscillator (which drives oxidation–reduction cycles of reactive oxygen species and lipids), two circadian timing systems that regulate behavioral and physiological processes according to a 24-hour light/dark cycle, can cause metabolic imbalances leading to fatty liver, dyslipidemia, glucose intolerance and an increased risk of cancer. However, little is known about how disruption of the intrinsic clock mechanistically alters metabolic pathways.
In a recent paper published in the Journal of Biological Chemistry, Natalia Monjes and colleagues at the Universidad Nacional de Córdoba in Argentina describe their study that found that disruption of the Bmal1 gene, a key component of the molecular clock, dampened temporal patterns in lipid metabolism of tumor cells compared to control cells. This dampening of lipid processes was accompanied by severe decreases in endogenous triglyceride levels, lipid droplet accumulation and reactive oxygen species content. The authors also observed an increase in lactate levels, which could indicate a Warburg effect–like hypermetabolic state.
These results not only confirm the phenomenon of serum-synchronized molecular cycling in HepG2 cells but also indicate an effect of this cycling on lipid biosynthesis and, in particular, on the ratios of specific phospholipids. These findings also highlight a metabolic susceptibility of tumor cells to circadian disturbance, which could be used to improve chronotherapeutic efficacy.
Modifying actin assembly dynamics
The assembly and disassembly of the actin network is crucial for regulating many cellular processes, including cell motility, cell division and intracellular transport. The diversity of these actin networks is the result of a multitude of remodeling proteins and posttranslational modifications, which can fine-tune actin fiber nucleation and elongation. However, researchers do not know yet how N-terminal posttranslational modifications such as acetylation and arginylation, which may constitute only a small percentage of actin at the leading edge of cells and filopodia, can affect the properties of actin.
Samantha Chin at Washington University in St. Louis and an international team used a method known as “pick-ya-actin” to produce pure populations of acetylated and arginylated actin (Ac-actin and R-actin, respectively) to compare their contributions directly to actin network dynamics, and they describe this work in a recent paper in the Journal of Biological Chemistry. Using pyrene-bound actin (which fluoresces upon polymerization) and total internal reflection fluorescence microscopy, they showed that Ac-actin exhibits higher spontaneous nucleation than R-actin, and that R-actin exhibits reduced elongation and branching compared to Ac-actin. The authors found no difference in cofilin-mediated severing of Ac-actin and R-actin strands, suggesting the effects of these modifications are primarily on assembly rather than disassembly kinetics.
These data begin to highlight an emerging role for N-terminal acetylation and arginylation of actin in the regulation of actin networks.
How Lyme disease evades the immune system
The complement cascade is a primary arm of the innate immune system, consisting of sequential protein cleavages that result in microbial death. However, some bacteria, such as Borreliella burgdoferi, which causes Lyme disease, have evolved outer surface-localized lipoproteins that help them evade complement-mediated attack. Researchers have identified two such proteins, termed ElpB and ElpQ, but have yet to understand fully the mechanism by which they inhibit the complement cascade.
In a follow-up study by Ryan Garrigues of East Carolina University and colleagues, published in the Journal of Biological Chemistry, the authors used multiple binding assays to show that the C-terminal domains of these Elp proteins were able to bind to complement protein C1s and block subsequent cleavage of the next sequential complement protein, C4. Furthermore, they found that this binding did not compete with C4 at the enzyme’s active site but rather occurred at an activation-induced binding site called an exosite.
Based on these results, the authors propose a model in which ElpB and ElpQ exploit activation-induced conformational changes in C1s that normally would promote C4 cleavage to prevent this reaction and thereby inhibit the complement cascade. This study shows a novel molecular mechanism employed by Lyme disease spirochetes to evade immune attack.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
Cracking cancer’s code through functional connections
A machine learning–derived protein cofunction network is transforming how scientists understand and uncover relationships between proteins in cancer.

Gaze into the proteomics crystal ball
The 15th International Symposium on Proteomics in the Life Sciences symposium will be held August 17–21 in Cambridge, Massachusetts.
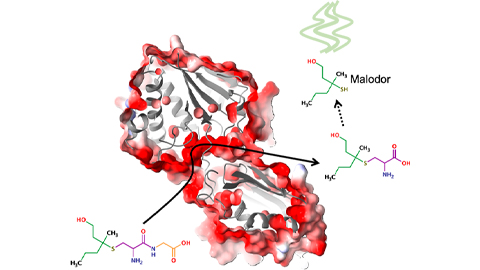
Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.

Neurobiology of stress and substance use
MOSAIC scholar and proud Latino, Bryan Cruz of Scripps Research Institute studies the neurochemical origins of PTSD-related alcohol use using a multidisciplinary approach.
Pesticide disrupts neuronal potentiation
New research reveals how deltamethrin may disrupt brain development by altering the protein cargo of brain-derived extracellular vesicles. Read more about this recent Molecular & Cellular Proteomics article.

A look into the rice glycoproteome
Researchers mapped posttranslational modifications in Oryza sativa, revealing hundreds of alterations tied to key plant processes. Read more about this recent Molecular & Cellular Proteomics paper.

