MCP: Infant gut microbes’ thirst for milk glycoproteins
Milk is a complicated liquid. Among its ingredients, which include lactose, lipids, free oligosaccharides and proteins, the free oligosaccharides perform the essential prebiotic roles of stimulating the growth of beneficial bacteria and preventing harmful bacteria from binding to epithelial cells in the gut. In a paper recently published in Molecular & Cellular Proteomics, researchers at the University of California, Davis, report that the bacteria in infants’ guts are also capable of digesting glycoproteins.
.jpg)
The researchers previously had thought of glycoproteins as a source of carbohydrate-bound amino acids that were only consumed by the infant, “but it turns out that parts of it, particularly the oligosaccharide, may also be feeding the microbiota,” explains Carlito Lebrilla, the paper’s senior author. “It has a dual use here — to give nutrition to the host, but also to enhance the microbiota.”
The new paper is a unification of sorts for the researchers, who have focused on the oligosaccharide components of human milk for a number of years and had an interest in the interactions between the microbiota and oligosaccharides.
While there was a good deal of research involving the milk oligosaccharides, Lebrilla explains, he and his colleagues hadn’t scrutinized what happens after the milk oligosaccharides interact with the microbiota. “This is one of the first times we’ve looked … in deep structural detail at what happens to the actual compounds as they go through” the gut, he says.
To determine the effects of digestion on the oligosaccharide portions of glycoproteins in breast milk, the researchers collected breast milk and stool samples from mother–infant pairs enrolled in the university’s Foods for Health Institute Lactation Study. They then isolated and cultured the dominant bacterial subspecies of Bifidobacteria longum from the stool samples. B. longum, in aggregate, accounts for up to 90 percent of the gut microbiota in milk-fed infants.
The investigators fed the bacteria the milk glycoproteins extracted from the breast milk. After demonstrating that the bacteria were breaking down the glycoproteins, Lebrilla’s group obtained a detailed profile of the oligosaccharides that had been released from the glycoproteins by subjecting the fecal samples to tandem mass spectrometry. The researchers were surprised to find the presence of degraded N-glycans in the fecal samples. N-glycans had been hypothesized as digestive targets because they were present in breast milk, but until now, they had not been observed post-digestion. The finding by Lebrilla and colleagues indicates that the bacterial species act upon the oligosaccharide components of the compound, which suggests the sugar moieties on breast milk glycoproteins can fuel the gut microbiome.
A collaborating group led by David Mills at the university’s Foods for Health Institute then sequenced the bacteria to get a comprehensive look at which enzymes were responsible for breaking down the milk oligosaccharides and when they were expressed, as well as their specificity. They found that one of the subspecies of B. longum contained genes for a glycoprotein-cleaving endoglycosidase. The researchers also noted that an exogalactosidase, BLNG_00015, was present in another dominant B. longum subspecies and capable of degrading glycoproteins with a terminal monosaccharide galactose residue. This, Lebrilla explains, is evidence of the specificity of the microbial enzymes for the glycoproteins.
Future work for Lebrilla and his colleagues will include examining the milk proteins that are being deglycosolated, exploring why certain milk protein concentrations seem to confer greater protection against stunting in infants than others, and investigating how the microbiota change in humans from a milk-oriented micobiota to a plant-based microbiota as we age.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
When oncogenes collide in brain development
Researchers at University Medical Center Hamburg, found that elevated oncoprotein levels within the Wnt pathway can disrupt the brain cell extracellular matrix, suggesting a new role for LIN28A in brain development.
The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
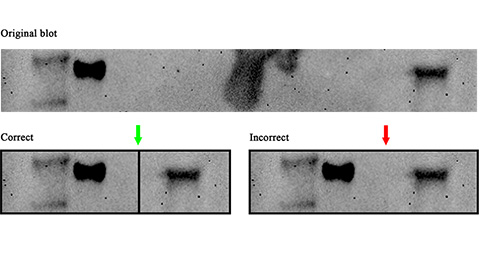
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

