From the journals: JBC
Dopamine-regulating oligomers. pH-dependent pathogen receptors. PIP2-mediated channel permeability. Read about papers on these and other topics in the Journal of Biological Chemistry.
pH-dependent recognition of pathogens
When pathogens invade the human body, sentinel macrophages called Langerhan cells that reside in tissues capture some of the pathogens, break them down and present antigens for adaptive immune cells. This swift activation of the adaptive immune system is essential for survival, and langerin, a receptor expressed on Langerhan cells, plays a central role in this process.
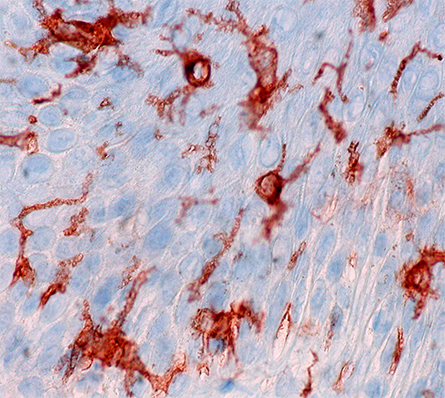
Langerin is a transmembrane carbohydrate receptor that belongs to the class of type II C-type lectin receptors. It recognizes a host of pathogens, including influenza virus, measles and HIV, by binding to carbohydrates on the pathogens’ surface. Central to langerin’s function is a calcium co-factor that resides in the carbohydrate binding pocket. After the pathogen is bound, langerin is engulfed by an endosome and degraded. When langerin and its bound pathogen are immersed into the acidic environment of the endosome, the pH change is sensed by protonation of the allosteric pH-sensor histidine H294. However, researchers do not yet understand the processes responsible for removal of the calcium co-factor from the binding pocket.
In a paper published in the Journal of Biological Chemistry, Jan-O Joswig of the Free University of Berlin and colleagues used molecular dynamics simulations and Markov models to find the molecular basis for the displacement of langerin’s calcium molecule and the subsequent release of its bound pathogen. The researchers showed that H294 protonation disrupts a precarious network of hydrogen-bonded protein residues, causing a conformational change. In this new conformation, a lysine side chain forms a new bond with a calcium-coordinating aspartic acid residue, resulting in calcium’s release. Once expelled, the calcium is prevented from rebinding through additional changes to the aspartic acid residue.
These findings show how biological systems use pH regulation of a binding site to drive a specific chain of functionally relevant conformational arrangements and may serve as a road map for future studies that aim to identify pH sensitivity in these systems.
Driving dopamine modulation
If asked to name a chemical responsible for brain functioning, most people would say dopamine is among the first that come to mind. Dopamine is central for a number of functions, such as reinforcement of behaviors, motivation, memory, attention and mood. Dopamine is released by brain cells into synapses, where it acts on the receptors of postsynaptic cells. While some of the unused dopamine then is degraded, most is taken up into the releasing cell by the dopamine transporter, or DAT. This process allows DAT both to recycle dopamine and to regulate the duration and intensity of dopamine-mediated neurotransmission. Thus, understanding how DAT functions can affect the design of therapies for a number of brain-related disorders. Researchers know that DAT molecules can oligomerize but do not yet understand the biological significance of this ability.
In recent work published in the Journal of Biological Chemistry, Tatiana Sorkina and colleagues at the University of Pittsburgh describe a series of small molecules that link transporter conformation to oligomerization and endocytosis. Using a combination of chemical cross-linking, fluorescence resonance energy transfer microscopy, an antibody-uptake endocytosis assay, live-cell lattice light sheet microscopy, ligand binding and substrate transport kinetics analyses, and molecular modeling and simulations, the authors showed that the DAT oligomerization and endocytosis induced by these small molecules involved interactions of four hydrophobic residues at the interface between two transmembrane helices. The authors then created a quadruple DAT mutant that replaced these same four hydrophobic residues and found that oligomerization and internalization of the receptor were suppressed. Moreover, the mutant DAT displayed altered dopamine transport kinetics and increased cocaine binding, suggesting that the residues involved in oligomerization also play an important role in the regulation of DAT function.
These findings show a direct coupling between conformational dynamics of DAT, functional activity of the transporter and the oligomerization leading to its internalization. They also highlight the identified transmembrane domains as a target for drug-mediated modulation of DAT activity.
PIP2 polices ion channel permeability
Transient receptor potential canonical type 5, or TRPC5, ion channels are calcium-permeable cation channels that are expressed in the brain and kidney. TRPC5 is involved in fear-related behaviors and also plays a role in chronic kidney disease, making it a promising therapeutic target. TRPC5 channels are activated transiently by phospholipase C enzymes, which hydrolyze phosphatidylinositol 4,5-bisphosphate, or PIP2, leading to phosphorylation of TRPC5 mediated by protein kinase C, or PKC. However, researchers do not understand yet how PIP2 and its product diacyl glycerol, or DAG, activate and maintain TRPC5 channel activity.
In a paper published in the Journal of Biological Chemistry, Mehek Ningoo and colleagues at Northeastern University distinguish between the processes responsible for channel activation and those underlying inhibition. Using whole-cell patch-clamp coupled with an optogenetic tool to dephosphorylate PIP2, the authors assessed channel–PIP2 interactions influenced by activators, such as DAG, or inhibitors, such as PKC phosphorylation, and used total internal reflection microscopy to quantify the channel cell surface density. They showed that PIP2 controls both the PKC-mediated inhibition and the DAG-mediated activation of TRPC5 currents by control of gating rather than channel cell surface density.
These findings may help researchers develop more selective and precise inhibitors to block TRPC5 channel activity, which might be used in therapies for chronic kidney diseases.
Dual-acting J-domain proteins aid chaperone client delivery
70-kDa heat shock proteins, or Hsp70s, are abundant molecular chaperones that are involved in nearly every stage of the cellular protein life cycle, from folding to remodeling, as well as in the development of diseases, including cancer. The efficacy and versatility of Hsp70 function rely on its cooperation with J-domain proteins, or JDPs, co-chaperones that stimulate the Hsp70 ATPase cycle and allow it to bind to client proteins.
In a recent study published in the Journal of Biological Chemistry, Hyunju Cho of the California Institute of Technology and colleagues showed that cytosolic JDPs have two distinct functions in the protein biogenesis process: initial capture of the client protein, in this case tail-anchored proteins, and transfer of the protein into the guided-entry-of-tail–anchored protein, or GET, pathway.
Using a hybrid protein containing yeast membrane protein that is strongly dependent on the GET pathway, specific mutations that compromised JDP function, and a battery of biochemical analyses including sedimentation and pulse-chase analyses, the authors discovered that the proteins Ydj1 and Sis1 function in parallel to support Hsp70-mediated relay of tail-anchored proteins to downstream chaperones so they can be delivered to the endoplasmic reticulum. To guide tail-anchored proteins into the GET stream, Hsp70s must capture and then transfer substrates to the protein Sgt2. The researchers showed that both of these actions are stimulated by JDPs in two independent steps involving ATP hydrolysis by Hsp70 and thus require at least two Hsp70 ATP cycles before the Hsp70 reaches the GET pathway.
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
How a gene spurs tooth development
University of Iowa researchers find a clue in a rare genetic disorder’s missing chromosome.
New class of antimicrobials discovered in soil bacteria
Scientists have mined Streptomyces for antibiotics for nearly a century, but the newly identified umbrella toxin escaped notice.
New study finds potential targets at chromosome ends for degenerative disease prevention
UC Santa Cruz inventors of nanopore sequencing hail innovative use of their revolutionary genetic-reading technique.
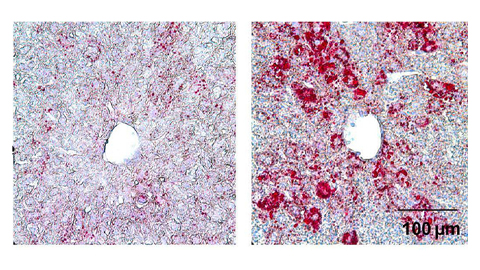
From the journals: JLR
How lipogenesis works in liver steatosis. Removing protein aggregates from stressed cells. Linking plasma lipid profiles to cardiovascular health. Read about recent papers on these topics.
Small protein plays a big role in viral battles
Nef, an HIV accessory protein, manipulates protein expression in extracellular vesicles, leading to improved understanding of HIV-1 pathogenesis.

Genetics studies have a diversity problem that researchers struggle to fix
Researchers in South Carolina are trying to build a DNA database to better understand how genetics affects health risks. But they’re struggling to recruit enough Black participants.

