DNA and vaccines
We have seen the terms “DNA” and “vaccine” used together in the media quite frequently in recent years due to the ongoing COVID-19 pandemic; in fact, Google reports anywhere from a 250% to 1,000% increase in searches in various combinations of these search terms.
So what is the deal with DNA, and does it play a role in vaccine development today?

A brief timeline and definition of vaccines
A vaccine is defined by the Centers for Disease Control and Prevention as a “preparation that is used to stimulate the body’s immune response against diseases.” In other words, vaccination exposes our body to a specific pathogen (a virus or bacteria that is harmful to us), giving our immune system a chance to survey it without extreme consequences of becoming ill.
During this surveillance period, specific immune cells, called B cells, produce antibodies that match unique molecular signatures (called antigens) on the pathogen or pathogen-derived proteins; this marks the pathogen or pathogenic proteins for destruction by other immune cells. The instructions to make antibodies specific to their antigen pairing are stored in B cells’ memory for possible future infections.

Infectious pathogens or pathogenic proteins do not stay in our bodies long, as many immune cells, such as T-cells and macrophages, recognize the antigen-antibody markings and subsequently degrade them to inhibit further spread of infection in the body. Also, B cells do not always remember the antigens from the pathogen the first time around or recognize slight differences (mutant forms) between antigen markings. This is why there is often a need for booster vaccines to keep up with mutant versions of a pathogen. The recipes for flu vaccines, for example, change on a yearly basis based upon epidemiologists’ best guess which strains will be most prevalent.
While doctors of the early 900s to 1700s may have not understood the scientific underpinnings of immunology and disease like we do today, they did utilize the scientific method to learn to provoke immunity.
Specifically, scientists discovered that exposure to infectious pathogens in small dosages primed human immune systems just enough that people recovered if they were exposed naturally later. This technique was termed “variolation” and was used to immunize patients against smallpox: Doctors exposed people to the contents of pustules from milder forms of the disease.
Borrowing from this idea in the late 1700s, English doctor Edward Jenner thought to instead use cowpox, a virus similar to the smallpox. In 1796, he tested it on a small boy, who made a full recovery, marking a breakthrough. Because the pus was from a cow-related disease, the new term for this type of exposure-infection-recovery system was “vaccination.”
The central dogma of biology and recent advances in vaccine technology
Today, when most people think about vaccines, what comes to mind are formulations in which the pathogen’s antigen is either a weakened or inactivated virus (like Jenner’s work) or a portion of a viral protein (such as the hepatitis B vaccine).
The race to slow the spread of COVID-19 led to the development and premiere of the first FDA-approved vaccines utilizing mRNA technology, but the concept of vaccines composed of primarily nucleic acid–based technology are not a novel or new concept. To understand why, let’s take a step back into the timeline of molecular biology and advances in vaccine development.

In a cell, proteins are naturally produced through the process of transcription and translation. Specifically, DNA stored in the nucleus holds a code that can be transcribed to mRNA (or messenger RNA). Because proteins can be translated to their final form only from mRNA molecules, this transcription step from DNA to mRNA is super important. The process of going from RNA to a protein is called translation. These combined processes are known as the central dogma of biology and have been studied thoroughly by scientists over the past century.
Understanding these processes, scientists thought that they could borrow from nature to create better vaccines. This led to advances in what is called recombinant technology, where “recombinant” is a scientific mechanism of copy and paste.
To make protein-based vaccines using recombinant technology, scientists took the DNA code for a portion of a pathogen’s protein surface and cloned (or transferred) it into another source, such as a plasmid DNA for bacteria or yeast. Then, the protein was produced by the natural transcription and translation machinery in these microbes; this allowed the protein to be produced in large quantities for vaccine production.
While this vaccine method (as well as older methods, such as those using dead or inactivated pathogens) are effective in the immunization process, they require a lot of work in their development and production to scale up to volume necessary to inoculate the public; this becomes a difficult factor to consider when a virus mutates and a new vaccine has to be produced quickly.
So, scientists decided to once again borrow from the central dogma of biology — but this time with a different kind of recombinant technology in mind.
Rather than cloning a DNA fragment into a plasmid for yeast and bacteria to produce it as a protein, scientists wanted to put a viral protein’s DNA code (or gene) into a vector that could be directly inserted into humans via vaccination. One way of accomplishing this was transferring the DNA into what is called the adenovirus, or the common cold virus-vector. With this technology, human machinery could produce enough protein to stimulate the immune system to prevent future disease, overcoming the production issues related to protein-based vaccines.
In the process of production, these vectors are also genetically engineered (or altered) so that the adenovirus itself cannot replicate or integrate into your DNA, once the vaccine itself is administered.
These types of vaccines are advantageous in that they provide the body with an antigen to target for antibody production with few side effects — all you feel symptomwise is similar to the common cold. This also means they can be given to immune-compromised individuals.
The Janssen (Johnson and Johnson) and AstraZeneca COVID-19 vaccines are based exactly on this technology. They were built upon earlier models for other diseases such as Ebola, tuberculosis and Middle East respiratory syndrome, or MERS, which is caused by a coronavirus.

mRNA vaccine technology
Also inspired by previous coronavirus and MERS outbreaks were the mRNA-based vaccines currently produced by Pfizer and Moderna.
The thought behind this type of vaccine design to simplify the work of recombinant adenovirus-vector vaccines by injecting the genetic code for the antigen directly as a piece of mRNA (and not as a vector).
This vaccine technology is quite convenient for cells, as it streamlines production of the antigen by cutting down on the process of transcription; instead, the RNA that enters a cell can be directly translated into a protein with antigen markings. This allows the immune cells recognize the protein as foreign and attack it.
And what’s even better: The RNA isn’t able to replicate itself and is subject to the cell’s machinery that naturally breaks down our own RNA. This RNA also is not be able to enter the nucleus, where our DNA is stored, and thus does not integrate into our DNA.
While the RNA-based COVID-19 vaccines are the first of their kind, they come with their own set of challenges.
A main barrier for worldwide production of these vaccines concerns their storage and expiration: Because RNA is a single strand of nucleic acid (and not a double helix like DNA), it is often more unstable than DNA above certain temperatures and cannot keep long. If the vaccine is kept out at room temperature for a long time prior to immunization, the person receiving the vaccine gets broken-down portions of the RNA, which are not sufficient code to translate the protein for the immune system to recognize and attack. Thus, countries without proper storage cannot benefit from this type of vaccine.
DNA-based vaccines
DNA-based vaccines are also not new.
They are used in veterinary settings for treating West Nile virus in horses and melanoma in canines, and clinical trials of therapeutic DNA vaccines for humans, such as those targeting various forms of cancer, are under way in the U.S.
Despite the ease in manufacturing these types of vaccines, the remaining challenge lies in their mechanism of delivery to cells. Because a DNA-based antigen needs to not only penetrate the cell membrane but also the nucleus, where replication machinery is housed in our cells, a simple stick with a needle (which is termed a “shot” colloquially) will not deliver DNA to the proper place in the body.
A recent Nature Biotechnology news article captured much of the ongoing research on delivery systems for these types of vaccines. Inovio Pharmaceuticals’ electroporation method involves applying an electric field to the injection site, causing the pores of the surrounding cell membranes and the pores of the nuceli to widen and allow the DNA molecules to pass across. Another delivery system, by Zydus Cadila, is the Tropis device. It involves a “pressurized jet of liquid, powered by a simple spring mechanism, to puncture the skin and deliver the vaccine intradermally.” It was recently utilized in India in the first-ever approved DNA-based vaccine, for COVID: ZyCoV-D.
Despite varied success, the issue with both of these delivery methods is their high cost. Researchers in Canada and the U.S. are hoping to change this, however, by developing cheaper devices or even device-free delivery.
DNA-based vaccines certainly have their advantages.
They are proving to be effective at preventing symptomatic COVID infection. ZyCov-D has 67% effectiveness, even with the delta variant.
Also, DNA is generally safe to store at room temperature with little degradation, thus allowing for widespread accessibility of the vaccine without concerns for the cost of storage.
Finally, because viral mutations vary considerably in terms of infection and death rates, having a DNA vaccine that is cheap and quick to produce would revolutionize our ability to respond to future outbreaks and maybe even help us prevent pandemics altogether.
As we reflect on how far vaccine technology has come on DNA Day 2022, don’t neglect to appreciate the power and potential of DNA vaccines.
Want to get your lab involved in DNA Day outreach? Check this link for suggestions of both in-person and virtual activities for 2022!
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
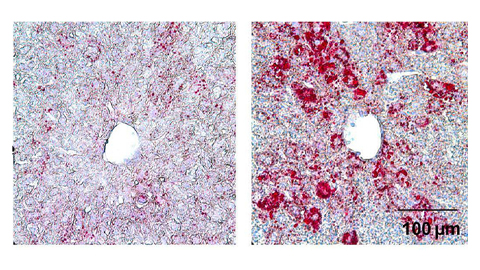
From the journals: JLR
How lipogenesis works in liver steatosis. Removing protein aggregates from stressed cells. Linking plasma lipid profiles to cardiovascular health. Read about recent papers on these topics.
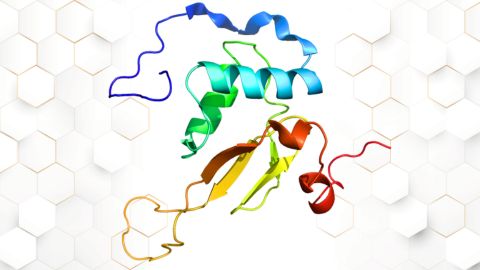
Small protein plays a big role in viral battles
Nef, an HIV accessory protein, manipulates protein expression in extracellular vesicles, leading to improved understanding of HIV-1 pathogenesis.

Genetics studies have a diversity problem that researchers struggle to fix
Researchers in South Carolina are trying to build a DNA database to better understand how genetics affects health risks. But they’re struggling to recruit enough Black participants.
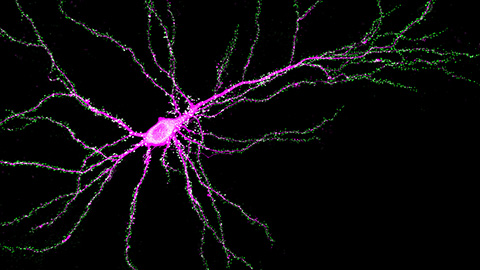
Scientists identify new function of learning and memory gene common to all mammalian brain cells
Findings in mice may steer search for therapies to treat brain developmental disorders in children with SYNGAP1 gene mutations.
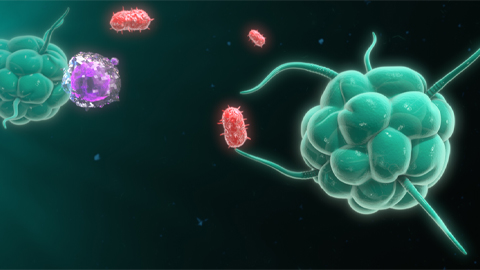
From the journals: JBC
Biased agonism of an immune receptor. A profile of missense mutations. Cartilage affects tissue aging. Read about these recent papers.
Cows offer clues to treat human infertility
Decoding the bovine reproductive cycle may help increase the success of human IVF treatments.


