Finding novel drug targets in tuberculosis
Even 27 years since the World Health Organization declared the “white plague” a global emergency, about 1.5 million people still die from tuberculosis each year. It is estimated that around one-fourth of the world’s population is infected with this bacterial pathogen. While many cases are latent, tuberculosis can complicate other health conditions, such as HIV and diabetes, which also complicate tuberculosis treatment. Preliminary data from a small study, not yet peer reviewed, suggest that TB may also increase patients’ susceptibility to COVID-19.

Our research team, based in New Zealand and funded by the Royal Society of New Zealand’s Marsden Fund, recently reported a novel mechanism of controlling synthesis of the essential vitamin K2 in the bacterial pathogen that causes tuberculosis. Our study was chosen as an Editors’ Pick by the Journal of Biological Chemistry.
We found that an enzyme called MenD from an early step in the process of making vitamin K2 is controlled by one of the metabolites produced at a later step, just before the vitamin K2 molecule is completed. Too much or too little vitamin K2 is toxic to the bacterium; this feedback inhibition allows the pathogen to slow down or speed up production of the vitamin.
As far as we know, this is the first time that allosteric regulation has been reported for the menaquinone (vitamin K2) biosynthesis pathway in any domain of life.
The ability to control vitamin K2 levels is likely to help the pathogen to adapt to the changing, sometimes hostile, environment in the human host during infection. Since humans do not produce vitamin K2, the enzymes that work together to produce it in bacteria may represent targets for new antimicrobials. Our discovery of this regulation site and its structure provide a potential new target for selective drugs.
Our team includes three midcareer researchers with young families, all affiliated with the Maurice Wilkins Centre for Molecular Biodiscovery. With colleagues Ghader Bashiri and Esther Bulloch from the University of Auckland, my laboratory at the University of Canterbury plans to work to unravel more mysteries about microbial pathogens. With support from the Canterbury Medical Research Foundation and the Maurice Wilkins Centre, my lab is already tackling work on metabolic pathways in the bacteria Staphylococcus aureus, which causes a difficult-to-treat infection in healthcare settings.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles
When oncogenes collide in brain development
Researchers at University Medical Center Hamburg, found that elevated oncoprotein levels within the Wnt pathway can disrupt the brain cell extracellular matrix, suggesting a new role for LIN28A in brain development.
The data that did not fit
Brent Stockwell’s perseverance and work on the small molecule erastin led to the identification of ferroptosis, a regulated form of cell death with implications for cancer, neurodegeneration and infection.

Building a career in nutrition across continents
Driven by past women in science, Kazi Sarjana Safain left Bangladesh and pursued a scientific career in the U.S.
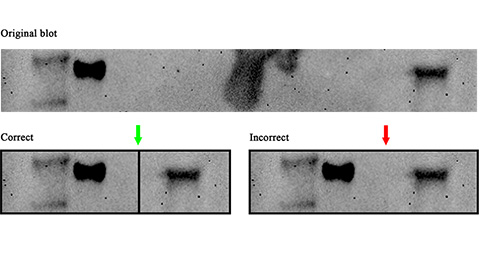
Avoiding common figure errors in manuscript submissions
The three figure issues most often flagged during JBC’s data integrity review are background signal errors, image reuse and undeclared splicing errors. Learn how to avoid these and prevent mistakes that could impede publication.
Ragweed compound thwarts aggressive bladder and breast cancers
Scientists from the University of Michigan reveal the mechanism of action of ambrosin, a compound from ragweed, selectively attacks advanced bladder and breast cancer cells in cell-based models, highlighting its potential to treat advanced tumors.
Lipid-lowering therapies could help treat IBD
Genetic evidence shows that drugs that reduce cholesterol or triglyceride levels can either raise or lower inflammatory bowel disease risk by altering gut microbes and immune signaling.

